Abstract
Background: Having depression and living in a rural environment have separately been associated with poor diabetes outcomes, but there little is known about the interaction between the 2 risk factors. This study investigates the association of depression and rurality with glycemic control in adults, as well as their interaction.
Methods: This is a repeated cross-sectional study with data collected from 2010 to 2017 (n = 1,697,173 patient-year observations), comprising a near-complete census of patients with diabetes in Minnesota. The outcome of interest was glycemic control defined as hemoglobin A1c under 8%. We used a logit model with clinic-level random effects to predict glycemic control as a function of depression, patient rurality, and their interaction, adjusted for differences in observed characteristics of the patient, clinic, and patient’s neighborhood.
Results: Having depression was associated with lower probability of achieving glycemic control (P < .001). Although rurality alone had no association with glycemic control, significant interactions existed between depression and rurality. Living in a small rural town mitigated the negative association between depression and glycemic control (P < .001).
Conclusion: Although patients with depression had poorer glycemic control, living in a small rural town reduced the negative association between depression and glycemic control.
- Cross-Sectional Studies
- Diabetes Mellitus
- Depression
- Glycated Hemoglobin A
- Hyperglycemia
- Logistic Models
- Minnesota
- Risk Factors
- Rural Health Services
Depression and living in a rural area have separately been associated with lower levels of achieving glycemic control in patients with diabetes, but we know little about the interaction between these 2 risk factors. This study leverages a rare opportunity to observe glycemic control across a large and heterogeneous mix of patients and primary care practices to close this gap in the literature by estimating the association and interactions of depression and living in a rural area with glycemic control in adults. An understanding of factors contributing to diabetes outcomes is critical to managing this increasingly prevalent condition. In the United States, 1 in 9 adults has diabetes and this could increase to 1 in 5 by the year 2025.1 The 2017 prevalence of diabetes in Minnesota was 7.8% of adults, approximately 330,000 people; approximately 18,000 new cases are diagnosed each year.2
Like diabetes, depression is a common chronic condition. It has been estimated that 7.1% of US adults had a major depressive episode in the past year.3 High rates of depression are a problem for diabetes care, since the odds of depression doubles for patients with diabetes compared with those without.4 Comorbid diagnoses of both diabetes and depression have been associated with lower levels of glycemic control,5 and have been associated with a decrease in quality of life, increased health care use and cost, increased disability, lost productivity, and an increased risk of death.6
Living in a rural area has previously been demonstrated to impact diabetes outcomes. Rural patients were less likely than urban patients to achieve glycohemoglobin A1c (HbA1c) below 7%,7 and were less likely to receive treatment for their disorder.8 In addition, rural patients relied on pharmacotherapy more than psychotherapy for their depression.9 Those with depression may face barriers to treatment in rural areas because of long travel times, poverty, stigma, lack of anonymity, culture of self reliance, and lack of culturally acceptable treatments, thereby potentially magnifying problems of achieving diabetes control.10
Since 27% of Minnesotans11 and 19% of the US population12 live in a rural area, it is important to learn how living with both depression and diabetes affects the likelihood of controlling these conditions for rural patients. Our research was done as part of the Understanding Infrastructure Transformation Effects on Diabetes (UNITED) project. In this study, we aimed to explore how depression and living in a rural area was associated with diabetes outcomes, specifically glycemic control.
In 2008, the Minnesota Legislature passed legislation mandating annual reporting of quality of care data by physician practices across the state, including measures for adult patients with diabetes.13 All Minnesota primary care and endocrinology practices treating 30 or more patients with diabetes are required to submit quality reporting data annually for diabetes. The Minnesota quality standards define glycemic control as an HbA1c less than 8% measured in the observation year. An HbA1c value between 7% and 8% is recommended by the American College of Physicians for patients with comorbid conditions.14 The objective of this study was to determine the combined effect of depression and living in a rural area on the achievement of the glycemic target of HbA1c < 8% in adults aged 18 to 75 years. We hypothesized that 1) depression, 2) living in rural areas, and 3) the interaction between depression and living in a rural area would all be associated with a lower probability of achieving glycemic control.
Methods
Data
The Minnesota Department of Health contracts with MN Community Measurement (MNCM), a nonprofit organization, to manage the quality reporting.15 For 2017 encounters, the quality reporting for diabetes was done through electronic data submission from 604 primary care and endocrinology practices in 100 parent medical groups. MNCM provided the patient-level diabetes data collected from primary care practices for use in our study. Our data contained HbA1c values for 1,697,173 patient-year records collected from 2010 to 2017, an average of 212,000 patients per year. This sample of adults aged 18 to 75 years includes the majority of Minnesotans with diabetes in this age range (currently estimated to be 330,000 across all ages).2 Because the encryption scheme for patient IDs changed annually, we could not group together records that were repeated observations of the same patient over different years. Therefore, we treated this as repeated cross-sectional data; the statistical implications of this treatment are discussed below. This study was reviewed and approved by the University of Minnesota’s Institutional Review Board.
Our dependent variable was glycemic control, defined as HbA1c < 8%. Our independent variables of interest were depression status and patient’s residential location. Practices submitting these data provided an indicator of patient’s current depression status. MNCM’s data submission guidelines recommend the Major Depression or Dysthymia (DEP-01) Value Set to define depression, but accept any documentation of a new or existing diagnosis of depression during the measurement period. Patient ZIP codes were mapped to Rural-Urban Commuting Areas (RUCA), and summarized to a 3-category variable of residential location describing where the patient lived 1) urban area, 2) large rural town, or 3) small rural town.16 RUCA coding examines both population density and commuting patterns to determine classification.
Patient-level covariates included: age, sex, diagnosis of ischemic vascular disease (IVD), diabetes type, and type of insurance coverage (commercial, Medicare, Medicaid, dual Medicare/Medicaid, self-pay, and unknown coverage). The indicator of IVD is provided by the practice. MNCM’s data submission guidelines recommend the Ischemic Vascular Disease Value Set to define an IVD diagnosis, but accept any documentation of a new or existing diagnosis in the measurement or prior years. We formatted patient age as a categorical variable to allow for nonlinear effects (18 to 44 years, 45 to 54 years, 55 to 64 years, 65 to 75 years). Because the data from MNCM do not include patient socioeconomic variables, we used 5-year average American Community Survey (ACS) data (2011 to 2015), mapped to patient ZIP codes, to describe the neighborhood in which the patient lived. From these data we created a neighborhood race/ethnicity variable (percent of population non-Hispanic White), a neighborhood poverty variable (percent of households with income under the federal poverty level) and the distribution of educational achievement in the neighborhood (percent of adults age 25 or greater with less than high school diploma, high school diploma or GED but no 4-year college degree, or a 4-year college degree).
Practice-level covariates included an indicator of Minnesota certification as a patient-centered medical home. In addition, we included the size of the medical group that owned the primary care practice. Size categories included single-site practices, practices in small medical groups (owning 2 to 11 primary care practices) and practices in large medical groups (owning 12 or more primary care practices).
We excluded records for patients with care managed by an endocrinology practice (85,058 records) because MNCM provided data only for locations reporting primary care providers onsite, in keeping with the goals of the UNITED project. Thus, we could not assume we had a representative sample of endocrinology practices in the state. In addition, endocrinology practices are rare in rural areas, so including patients reported by endocrinology practices might bias our results. We also excluded records for patients with missing data (19,333 records). Missing data were almost exclusively from incomplete matches with RUCA and ACS data. Together, eliminating data reported by endocrinology practices and records with missing data reduced our 8-year sample from 1,801,564 to 1,697,173 records, a 5.8% reduction in sample size. Because we restricted our sample to those with care managed by a primary care practice, patients in omitted records tended to be younger, with an associated higher prevalence of type 1 diabetes and less Medicare coverage.
Statistical Analysis
We modeled achievement of glycemic control (HbA1c < 8%) in a logit framework as a function of depression and residential location, and the interaction between having depression and living in a rural area. We estimated a baseline model without this interaction to provide a direct comparison with the existing literature that tested association between glycemic control and depression, or glycemic control and rurality. The logit regression models were adjusted for annual trend as a continuous variable, and the patient-, practice- and neighborhood-level characteristics described above. We included practice-level random effects to capture unobserved time-invariant practice and patient characteristics. We conducted the statistical analysis with Stata 15 statistical software using α = 0.05 as the cutoff for statistical significance.17
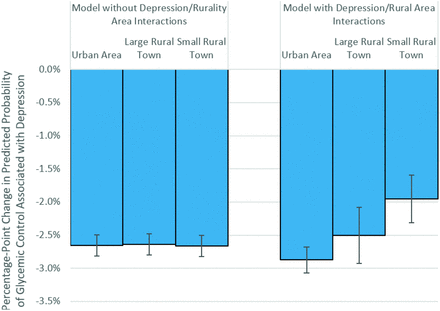
A positive coefficient from our logit regressions indicates an increase in the probability of glycemic control as that coefficient’s variable increases, but does not tell the reader how large that increase in probability is. For this reason, logit results are often presented as odds ratios to give the reader a more intuitive understanding of the change in likelihood of the outcome. But in our results, we have the added complexity of interaction terms, a complexity that is not addressed by a simple transformation from coefficients to odds ratios. Therefore, to illustrate the impact of a change in depression status on the probability of glycemic control, we estimated the average percentage-point difference in the probability of glycemic control with and without depression, presented below in Figure 1. In other words, the models were used to compute the average expected change in the probability that HbA1c < 8% when depression status changes from “no depression” to “depression.” This was done multiple times, holding residential location fixed at each of the 3 possible categories (urban area, large rural town, small rural town), to demonstrate how these changes in probability were affected by residential location. All other patient, practice and neighborhood characteristics were held at their true value. A negative percentage-point change in probability would indicate that glycemic control was predicted to get worse with depression diagnosis.
Predicted change in the probability of achieving HbA1c < 8% associated with change in depression status by residential location. Note: Predicted changes in probabilities are based on the multivariate logit models. The models are used to compute the average expected change in the probability that HbA1c < 8% when patient depression status changes from “no depression” to “depression,” assuming the residential location is fixed at the level described and all other patient characteristics are held at their true value. The negative changes in probability indicate that glycemic control worsens when a patient is depressed, though the level of this decline may vary by patient location. Error bars indicate the 95% CIs for the estimated changes in probability.
Results
There were 1,697,173 total patient-year records analyzed in the 8 years of observation from 2010 to 2017 (Table 1). The average HbA1c value in our sample was 7.3% and the average rate of achieving an HbA1c < 8% was 76.1% (1,291,705 records). Only 100,045 (5.9%) records were for patients with type 1 diabetes. Patients had depression in 384,699 (22.7%) records, and 291,998 (17.2%) records indicated IVD. The sample was slightly more male than females (53.8% vs 46.2%). The majority of records (1,158,921, 68.3%) were from an urban area, 12.9% (219,667) from large rural towns, and 18.8% (318,575) from small rural towns. Averaging across the ACS statistics in our data, 82.3% of the residents in the patients’ neighborhoods were White and non-Hispanic. Most adults (mean, 60.9%) in these neighborhoods had a high school degree but had not completed a 4-year college degree, with a mean of 11.5% households living in poverty.
Sample Characteristics of Minnesota Adults with Diabetes Observed 2010 to 2017
In Table 1 we see that rates of depression varied by patient location in an inverted U-shaped pattern, highest in large rural towns. Patients in urban areas have higher rates of commercial insurance and Medicaid coverage, and lower rates of Medicare coverage, compared with patients living in rural areas. Patients living in urban areas were also more likely to receive care from a practice owned by a large medical group and tended to live in more racially diverse and more highly educated neighborhoods. These differences suggest that controlling for both observed patient- and practice characteristics, and unobserved time-invariant characteristics (using random effects), is important when comparing glycemic control across residential locations.
Our regression results are shown in Table 2 for models with and without the interaction of depression and living in a rural area. In the baseline model (no depression/rural area interaction), depression was associated with a lower likelihood of achieving HbA1c < 8% (coefficient –0.150, P < .001), though we found no statistically significant differences in glycemic control by residential location. We found similar results when we included an interaction term (depression coefficient –0.162, P < .001). Living in a rural area had no statistically significant main effect, but the interaction terms suggest living in rural areas had a protective effect against the depression impact, as described below. This was statistically significant in small rural towns (interaction coefficient 0.051, P < .001).
Logit Regression Coefficients for Glycemic Control (HbA1c < 8%) by Depression and Patient Location, Adjusted for Covariates at Patient, Practice, and Neighborhood Levels
Coefficients of important control variables are also listed in Table 2. Glycemic control increased with age and for females. Type 1 diabetes and IVD were associated with lower rates of glycemic control. The type of primary care practice made a difference. Practices had more patients with glycemic control if they were patient-centered medical home certified (coefficient 0.049, P < .001) or owned by a large medical group (small coefficient –0.087, P < .001; single site coefficient –0.104, P = .006).
In Figure 1, we illustrate the impact of a depression diagnosis on the probability of achieving glycemic control; all 6 effects noted below are statistically significant at P < .001. The baseline model included no interaction between depression and living in a rural area, so the impact of depression was estimated to be nearly identical across residential locations; in this baseline model we estimated that the presence of a depression diagnosis was associated with a –2.6 to –2.7% change in the probability a patient will achieve glycemic control across all locations, holding all other factors constant. In the model with interactions between depression and residential location, depression was associated with a –2.9% change in the probability of glycemic control in urban areas. In large rural towns, depression was associated with a –2.5% change in the probability of glycemic control. The difference between these urban and large rural town effects was not statistically significant. In small rural towns, depression was associated with –2.0% change in the probability of glycemic control, a statistically significant difference from the –2.9% change in urban areas (P < .001).
To provide insight into mechanisms driving differences in care patterns by residential location, we requested additional data from the UNITED project. The UNITED research team extracted counts of office visits for each patient for the time period 2008 through 2014, the date range for which patient location was available. These claims data were provided to the UNITED project by a local health plan for Minnesota patients with diabetes who were enrolled in the health plan’s commercial, Medicare and Medicaid products, comprising approximately 86,000 patients with diabetes each year. The summarized data are displayed in Table 3, showing higher annualized rates of office visits per patient in urban areas. A depression diagnosis was also associated with increased office visits. The depression-related increase in visits was stronger for specialty care, resulting in an increase in the fraction of office visits that were for specialty care in both settings, but this shift in distribution from primary to secondary care was higher in urban areas. Specifically, a diagnosis of depression was associated with a 5.1 percentage-point drop in primary care as a fraction of total visits in urban areas, with a smaller 2.4 percentage-point drop in rural areas.
Annualized Rates of Office Visit Encounters by Depression Status and Residential Location Observed in Health Plan Administrative Data 2008 to 2014
Discussion
The results of this study support our hypothesis that the presence of depression reduces the likelihood of glycemic control. Contrary to our expectations, our baseline model—excluding interactions between depression and living in a rural area—found no negative effect of living in a large or small rural town on the probability of glycemic control. The model that included interactions between depression and living in a rural area had a similar, statistically insignificant main effect of living in a rural area, but the interactions from that model showed that living in a rural area appeared to have a protective effect against the association between depression and worse glycemic control, also contrary to our expectations. Patients with depression living in a small rural town had a greater likelihood of achieving glycemic control than patients with depression in urban areas.
Our finding that depression reduced the likelihood of glycemic control is consistent with the results of prior studies.5,18 However, our finding that living in a rural area was not associated with worse glycemic control, and even mitigated the impact of depression, is not consistent with other studies that found that rural patients experienced difficulties achieving HbA1c targets.7 We expected access to health care to be a challenge for rural residents, inhibiting their ability to manage their diabetes. And in fact, in Table 3 we did find lower rates of office visits per patient in rural areas, and this rural-urban difference widened when a depression diagnosis was present, though this did not translate to worse glycemic control for rural areas. This pattern of decreased health care access in rural areas has been a national concern.19
Differences in health care delivery between rural and urban areas is 1 possible explanation for the protective effect of living in a rural area. In Minnesota, there is significantly greater access to physicians, particularly specialists, in urban areas, which have 376 physicians per 100,000 residents, relative to large rural towns (208/100,000) and small rural towns (101 per 100,000).20 In contrast with urban residents who have greater access to behavioral health specialists, patients in rural environments may have their primary care provider manage both their diabetes and depression.21 This pattern is consistent with a study using a focus group to explore the increased role that primary care providers in rural areas have in providing mental health services.21 Simultaneously treating both conditions may increase coordination of care and increase the probability of timely diabetes care because of the increased frequency of visits associated with depression care. The health plan data in Table 3 also support this theory. We found the fraction of office visits provided in primary care practices decreased when a patient had depression, but this shift to specialty care was larger in urban areas, suggesting diabetes and depression care are more frequently coordinated through primary care providers in rural areas.
The protective effect of living in a rural area may also be a by-product of more dense social networks.22 In a telephone survey of Louisiana residents (n = 1500), personal networks in rural areas have ties of stronger intensity.23 In another study of California residents (n = 1600), depressive symptoms are inversely associated with the size of social networks.24
Our predicted probabilities of depression’s impact on glycemic control ranged from a 2.9% decrease in urban areas to a 2.0% decrease in small rural towns (Figure 1). Relative to the average 76% rate of glycemic control in our population (Table 1), some may question the practical significance of this protective effect. However, small average differences translate to meaningful impacts at the population level. If we could identify the source of this mitigation of the depression effect and translate it to the patients in living in urban areas, back-of-the-pad calculations suggest we could see an additional 30,000 to 35,000 adult patients in glycemic control in the United States each year.
Limitations
Our analyses were constrained by data limitations, specifically our inability to track patients over time. But if unobserved patient characteristics were stable over time, the use of practice-level random effects should control for the temporal correlation caused by repeated observation of the same patient across time. An additional limitation of our study was the geographic restriction to Minnesota data. However, our sample comprised a near complete census of the population (age 18 to 75 years) with diabetes, came from a broad sample of primary care practices, and spanned every socioeconomic status and insurance coverage type in the state, increasing the likelihood that the findings have national relevance. Our broad patient sample, careful econometric methods, and controls for patient, neighborhood, and practice characteristics, increase the probability that our estimated associations between depression, living in a rural area, and glycemic control are generalizable beyond Minnesota.
One challenge to our analysis might be the inclusion of patients with type 1 diabetes, because of the significant differences in presentation of the condition and strategies for management of these chronic conditions. A subanalysis of only patients with type 1 diabetes found similar patterns by patient location, that is, no main effect of living in rural areas, but a mitigation of the impact of depression for patients living in rural areas. The only difference is that this mitigation of the impact of depression was much stronger for patients with type 1 diabetes, suggesting the results we present here are a lower bound for the impact of living in rural areas.
Conclusion
We know from previous studies that adults diagnosed with diabetes are at a higher risk for depression, and that comorbid diabetes and depression may make self care and wellness more difficult, leading to more uncontrolled diabetes. For this reason, a better understanding of the interaction between patient’s location and depression is important in improving outcomes for patients with diabetes. We found that living in a rural area appeared to exhibit a protective effect against the impact of depression on glycemic control. One possible mechanism for this protective effect may be differences in social networks between rural and urban areas. Another possibility may be an increase in simultaneous treatment of depression and diabetes by primary care physicians in rural areas, compared with fragmented treatment across specialties. Understanding the mechanisms of this protective effect in rural areas could improve our understanding of other contextual influences on patient care delivery.
Acknowledgments
HF, VGS, and CC wrote the manuscript and researched the data. AMR, LS, and KAP contributed to the general discussion before writing and reviewed and edited the manuscript.
Notes
This article was externally peer reviewed.
Funding: Research reported in this publication was supported by the National Institute of Diabetes And Digestive and Kidney Diseases of the National Institutes of Health under Award Number R18DK110732. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/33/6/913.full.
- Received for publication January 28, 2020.
- Revision received May 11, 2020.
- Accepted for publication May 18, 2020.