Abstract
Use of the lowest clinically effective dose of postmenopausal hormone therapy conforms to current recommendations and good clinical practice. Although accumulating evidence demonstrates the efficacy and tolerability of low hormone therapy doses, data about their use are limited by a lack of long-term, randomized studies. This review evaluates current evidence on the efficacy, safety, and tolerability of these preparations and their role in menopausal management.
The primary indication for postmenopausal hormone therapy (HT) is to relieve the vasomotor symptoms (hot flashes, night sweats) and vaginal dryness and discomfort that often accompany the menopausal transition and beyond. These symptoms, which are attributed to the natural decline in estrogen during and after menopause, can be remedied with HT. The loss of bone mineral density (BMD) and associated skeletal fragility and increased risk of fracture are also effectively mitigated by HT in postmenopausal women.1–3 In women with a uterus, a progestin is used to oppose estrogen-stimulated endometrial proliferation and protect against endometrial cancer.
The safety and tolerability of therapeutic agents must be considered in balance with their clinical efficacy. Reports from the Women's Health Initiative (WHI) trials raised concerns about venous thromboembolic events (VTEs), coronary heart disease (CHD), breast cancer, and stroke in women who were an average of 12 years postmenopause and received estrogen plus progestin (EPT) when compared with women who received placebo (Table 1).2,4–10 The estrogen-alone (ET) arm in women without a uterus found increased risks of thromboembolic events and stroke with treatment but no increase in CHD or breast cancer (Table 2).3,4,11–13 Both trials found reduced risks for major fracture and colorectal cancer, the latter particularly in women randomized closer to menopause.2,3 However, given that the WHI study population may have been at greater risk for adverse events by virtue of age and years since menopause, the relevance of WHI data to the management of the typical symptomatic woman has been questioned.
Consistent with the most commonly prescribed treatment options at the time the WHI trials were designed, HT doses of 0.625 mg conjugated equine estrogen (CEE) with or without 2.5 mg medroxyprogesterone acetate (MPA) were used. Publication of the initial results from the WHI EPT trial focused attention on the adoption of lower HT doses in clinical practice.14 Today, the use of lower starting doses for treatment of postmenopausal symptoms is increasingly recommended.14,15
Currently, oral doses as low as 0.3 mg/day of CEE and 0.5 mg/day of 17β-estradiol (E2) are available to treat menopausal symptoms, and transdermal patches are available with estradiol doses as low as 0.014 mg/day. The purpose of this article is to describe what is known about the efficacy, safety, and tolerability of the currently available lower-dose HT formulations as applied to their most common uses.
WHI: Beyond the Preliminary Results
After the early termination of the WHI trial of combination HT in 2002, initial results indicated that 5 years of standard-dose estrogen (0.625 mg CEE) plus progestin therapy decreased a woman's risk of fracture and colorectal cancer but increased her risk of VTE, CHD, breast cancer, and stroke.2 Although the average age of the participants in the WHI study population was 63 years at the time of study enrollment, with 74% naïve to HT, the overall risks of HT were said to outweigh the benefits in the study group. These findings were then generalized to all postmenopausal women. However, results from the ET arm of the WHI,3 the age-stratified analyses from the EPT arm of the WHI,4 and the Nurses’ Health Study16 demonstrated that the risk-benefit profile of standard-dose ET and EPT is more favorable in women closer to the menopausal transition compared with older women, the predominant population in the WHI.
Growing Interest in Lower Hormone Doses
Using the lowest effective dose of any therapy remains a fundamental tenet of clinical practice and a valuable goal in the treatment of postmenopausal women. Consequently, current guidelines unanimously recommend the use of the lowest effective HT dose.1,17,18 Available evidence suggests that lower HT doses may be better tolerated and have fewer adverse effects than standard doses. Using a lower estrogen dose may also reduce the dose of progestin needed for endometrial protection.15,19,20 As discussed below, accumulating clinical trial data demonstrate the efficacy of lower HT doses for several indications. However, it is important to acknowledge that the minimally effective dose may not be the same for all women, and that the lowest dose may not address all symptoms in one individual.
In recent years, numerous low-dose estrogen options have been introduced. Currently available low-dose estrogen formulations are listed in Table 3 and include oral CEE 0.45 and 0.3 mg/day; oral ethinyl estradiol 0.025 mg/day; oral esterified estrogens 0.3 mg/day; and transdermal E2 0.0375 and 0.025 mg/day. Low-dose estradiol is available as an oral formulation of E2 (0.5 mg/day). An ultra-low dose of transdermal E2 (0.014 mg/day) is also available, although this dose is approved only for the prevention of postmenopausal osteoporosis. Several combination low-dose oral EPT combinations are also available, including E2 0.5/norethindrone acetate (NETA) 0.1 mg/day; CEE 0.45/MPA 1.5 mg/day; and CEE 0.3/MPA 1.5 mg/day.
Low-Dose Estrogen Formulations
Efficacy of Lower HT Doses
The efficacy of lower HT doses has been investigated in many clinical trials; the consensus of these studies is that lower HT doses relieve vasomotor and vulvovaginal symptoms and prevent postmenopausal bone loss with improved tolerability compared with standard doses.14,15,21 Representative studies are reviewed below.
Vasomotor Symptoms
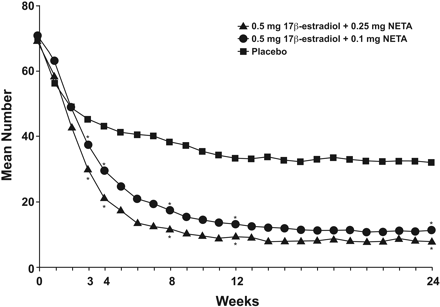
Most women who initiate HT do so to relieve menopausal hot flashes and night sweats. Considerable evidence indicates that lower doses of HT effectively relieve vasomotor symptoms (Table 4).22–30 A recent study of 425 women with at least 50 moderate-to-severe hot flashes per week found that low-dose transdermal estradiol (0.014 mg/day) was effective in reducing vasomotor symptoms compared with placebo.22 Oral doses as low as 0.3 mg daily of esterified estrogens were also effective.24 Studies of ET have typically demonstrated differences after approximately 8 to 12 weeks.25–31 The Women's Health, Osteoporosis, Progestin, Estrogen trial, which tested CEE at 0.625, 0.45, and 0.3 mg/day, alone and with MPA 2.5, 1.5, and 1.5 mg/day, respectively, demonstrated efficacy for all regimens compared with placebo.25 However, the rate of decline in vasomotor symptoms was slightly faster with the progestin-containing regimens. Another study that tested 2 progestin doses in an estrogen-progestin combination (E2 0.5/NETA 0.1 mg/day and E2 0.5/NETA 0.25 mg/day) found a significant decrease in the frequency and severity of hot flashes for each active treatment versus placebo after 3 weeks (Figure 1).28
The number of moderate-to-severe hot flashes, by week, with low-dose E2 0.5/NETA 0.25 mg, E2 0.5/NETA 0.1 mg, and placebo. *Significantly different from placebo (P = .001). NETA, norethindrone acetate. (Reproduced with permission from Panay N, et al. Ultra-low-dose estradiol and norethisterone acetate: effective menopausal symptom relief. Climacteric 2007;10:120–31.28)
Placebo-Controlled Trials of Low-Dose Estrogen Preparations in Hot Flash Relief22–30
In the trial of transdermal regimens reported by Bachmann et al,22 participants aged 40 to 71 years (average, 53 years) received 0.023 mg/day E2 with 0.0075 mg/day levonorgestrel, 0.014 mg E2, or placebo for 12 weeks. As mentioned above, E2 0.014 mg/day significantly reduced the number and severity of vasomotor symptoms compared with placebo (Figure 2).22 However, treatment response rates were somewhat greater with E2/levonorgestrel, suggesting that the concomitant use of a progestin or the slightly higher dose of estrogen provided an advantage in the relief of vasomotor symptoms.
Change from baseline in the mean weekly frequency of moderate-to-severe hot flushes with transdermal E2 0.014 mg/d, transdermal E2 0.023 mg/d plus levonorgestrel (LNG) 0.0075 mg/d, and placebo. *P < .001 vs placebo. (Reproduced with permission from Bachmann G, et al. Lowest effective transdermal 17β-estradiol dose for relief of hot flushes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2007;110:771–9.22)
Vulvovaginal Atrophy
Vasomotor symptoms usually decrease over time; however, vulvovaginal atrophy generally worsens with increasing duration of estrogen deficiency or with increasing time since menopause. Low-dose estrogen therapy has been shown to significantly reduce vaginal atrophy and its associated symptoms in clinical trials.25,28,29,32 In general, studies have shown that low-dose estrogen formulations provide greater improvement in vaginal epithelium than placebo but less than that observed with conventional doses of estrogen.32 In a recent trial of the impact of 2 low-dose formulations of E2 (0.5 mg/day with either 0.1 mg or 0.25 mg NETA) in a population of younger postmenopausal women who had a low baseline incidence of urogenital symptoms, both therapies resulted in a reduction in vaginal dryness scores and a statistically significant improvement in vaginal maturation and vaginal pH compared with placebo.28 Low-dose regimens of CEE with and without MPA also have been shown to result in statistically significant improvement in vaginal maturation indices.25
Local estrogen is also effective for treating urogenital symptoms associated with postmenopausal atrophy of the vagina and the lower urinary tract but they do not provide any vasomotor or osteoporosis benefit. Delivery options include an estrogen vaginal ring, which provides approximately 7.5 μg of estradiol per day for 3 months of treatment with each insertion; vaginal estradiol tablets, which provide 25 μg of estradiol per day; and CEE cream, which provides 0.625 mg/g.33–35 Vaginal estradiol tablets are administered daily for 2 weeks and twice weekly thereafter.35 The CEE cream is recommended for use in a cyclic regimen (daily for 21 days followed by 7 days without treatment) with reassessment at 3- to 6-month intervals. Local treatments differ from other low-dose options in that they do not provide any vasomotor or osteoporosis benefit. According to the North American Menopause Society, progestogen is generally not indicated with local administration of low-dose estrogen for vaginal atrophy.36 Data from several studies of low-dose local vaginal estrogen therapy have reported that the risk of endometrial proliferation without the concomitant use of a progestin is low.37,38 However, clinical surveillance for potential endometrial effects in women using local estrogen therapy is recommended, a guideline that reinforces the need for using the lowest effective estrogen dose.
Osteoporosis Prevention
The efficacy of low-dose HT in preventing postmenopausal bone loss has been the focus of numerous studies in recent years. Low doses of HT and lower doses of ET have been shown to effectively prevent bone loss in postmenopausal women.39–41 For example, lower doses of oral ET (0.25 and 0.50 mg/day) reduced bone turnover in healthy women older than 65 years of age to a similar degree as that seen with standard-dose therapy (1.0 mg/day).39
The impact of low-dose HT on BMD and bone turnover also has been evaluated in younger postmenopausal women at risk for rapid bone loss.40,41 In the 2-year osteoporosis substudy of the Women's Health, Osteoporosis, Progestin, Estrogen study, low doses of CEE alone (CEE 0.45 mg/day and CEE 0.3 mg/day) and CEE plus MPA (CEE 0.45, MPA 1.5 mg/day and CEE 0.3, MPA 1.5 mg/day) effectively increased BMD and total bone mineral content and reduced markers of bone turnover in postmenopausal women within 4 years of their last menstrual period.40 Addition of MPA 2.5 mg/day to CEE 0.625 or 0.45 mg/day increased spinal BMD in all groups compared with CEE alone.
Speroff et al41 also evaluated the impact of various low doses of ethinyl estradiol (EE) with or without NETA in women within 5 years of menopause. Regimens with EE/NETA produced significant dose-related increases in BMD that were greater than those observed with unopposed EE therapy.41 After 24 months, increases in BMD with EE/NETA ranged from 5% to 6% compared with 0% to 3% with unopposed EE.
In summary, low-dose HT provides effective protection against postmenopausal bone loss. The addition of a progestin seems to augment improvements in BMD.40,41
Tolerability and Safety of Lower HT Doses
Clinical trials have demonstrated that lower HT doses provide menopausal symptom relief and osteoporosis prevention with greater tolerability than standard HT doses. Preliminary observational evidence suggests that lower HT doses may also be associated with an improved long-term safety profile compared with standard doses; however, data from large long-term, randomized, controlled trials are lacking.
Endometrial Safety
The primary goal of progestin use in HT is to reduce the risk of endometrial hyperplasia and endometrial cancer. Progestogens can attenuate some of the favorable effects of estrogens on lipids,42 and they are associated with breast tenderness.43 Accordingly, the lowest effective dose of this component is important. Because there is a clear dose-response relationship between the dose of estrogen and the risk of adverse endometrial effects, the progestin dose required for adequate endometrial protection with lower ET doses has been well investigated in clinical trials. These trials demonstrate that lower estrogen doses, when used with adequate doses of progestin (which, in some regimens, are also lower than standard), have an excellent endometrial safety profile.19,20,41
However, some experts have suggested that older women using low doses of estrogen may not require the regular use of a progestin.15 In one study of postmenopausal women aged 60 to 80 years, participants who received unopposed transdermal estradiol (14 μg/day) had similar rates of endometrial hyperplasia, endometrial proliferation, and vaginal bleeding compared with participants who received placebo during 2 years of treatment.32 This may be related to the declining density of endometrial estrogen receptors with advancing age. In contrast, other investigators have noted an increased incidence of endometrial hyperplasia in younger women (aged 40–65 years) after 2 years of treatment with low-dose unopposed estrogen.20 Consequently, clinical practice guidelines recommend adding a progestin in women with an intact uterus.1,17,18 Although all progestins commonly used in HT provide effective endometrial protection, there is some evidence that micronized progesterone may be better tolerated and may exhibit a reduced proliferative effect on breast tissue compared with other progestins, such as MPA.44,45
Bleeding Profile
One of the most notable benefits of using lower HT doses versus standard doses is an improved uterine bleeding profile. Controlled trials of lower HT doses have reported higher rates of amenorrhea and lower incidences of vaginal bleeding compared with standard doses, particularly in the early cycles of therapy.39,46,47 Because the degree and frequency of bleeding is a strong predictor of patient continuation of HT,48,49 the reduced bleeding associated with low-dose HT may improve patient compliance and extend its benefits to more women.
Breast Health
Women using lower EPT doses have a reduced likelihood of experiencing breast tenderness, a relatively common side effect of standard-dose HT, compared with women using standard doses of therapy.25,39 The lower progestogen doses made possible by reduced estrogen doses may be important in this regard.25,39
The WHI found a modest increased risk of breast cancer in women who received standard-dose EPT, but not among women without an intact uterus who received unopposed estrogen (Tables 1 and 2).2,3 The increase with EPT was widely reported and the null finding with ET is not as well known; it is the rare patient who feels comfortable integrating this information for herself. Consequently, the relationship between lower doses of HT and breast cancer may be a subject of concern for many women.
The effect of lower doses of estrogen and/or progestin on breast cancer risk is unclear and currently there is minimal evidence that addresses this important clinical issue. A large US prospective observational study reported no increase in breast cancer risk with the use of CEE alone at doses of 0.3, 0.625, and 0.9 mg/day with durations of either <5 or ≥5 years; however, there was an inverse trend across the 3 dose levels that approached statistical significance (P = .06).50 Among users of combination CEE plus MPA therapy, there was no dose-related breast cancer risk seen for progestin, but daily progestin was associated with an increased risk whereas use for <2 weeks per month was not. In contrast, the Collaborative Group on Hormonal Factors in Breast Cancer's reanalysis of data from 51 studies representing nearly 53,000 cases and 108,000 controls did not find an effect of estrogen dose on breast cancer risk.51
There has been recent interest in the use of mammographic density as a predictor of breast cancer risk in women using HT because of a reported association between breast density and breast cancer.52 Whether breast density changes associated with HT affect breast cancer risk is a subject of debate.53,54 A recent study by Boyd et al53 directly addressed this question and found no support for this association. Data on more than 1700 postmenopausal women from 3 observational cohorts were combined and it was found that breast density on mammography did not mediate the relationship between HT and breast cancer, although breast density and HT were each independently associated with breast cancer.53 Consistent with the nearly universal findings that breast cancer risk is associated with current use of HT and that it declines to a rate comparable with never use after approximately 5 years,51,55 the purported association between HT, breast cancer, and mammographic density53 or abnormal mammograms7 may represent a surveillance effect rather than an etiologic pathway.56,57 Nonetheless, a recent study found that low-dose E2/NETA use had a neutral effect on mammographic breast density.58
Cardiovascular Effects
Venous Thromboembolism
An increased risk of VTE is a well-documented consequence of standard-dose HT.2,3 Although preliminary observational evidence indicates that lower HT doses may have a reduced effect on VTE risk compared with standard-dose therapy,59 additional research is needed to clarify the impact of lower doses of therapy among HT users. However, recent data indicate that VTE risk may be minimized or eliminated with the use of transdermal estrogen preparations, even at standard doses.60 Again, these findings need replication in experimental study designs but, if validated, low-dose transdermal HT could have a safety advantage in this domain.
Stroke
A statistically significant association between HT and stroke, principally in women aged 60 years and older, has been described in the WHI (Tables 1 and 2) and in observational studies.3,5,61–63 There are limited data about the risk of stroke with lower HT doses. However, a report from the Nurses’ Health Study found that the estrogen dose affected the risk of stroke associated with HT.61 In this analysis, CEE doses ≥0.625 mg/day were associated with a significantly increased risk of stroke compared with no therapy (relative risk [RR], 1.35; 95% CI, 1.08–1.68) whereas CEE dosed at 0.3 mg/day was not linked to an increase in stroke risk (RR, 0.54; 95% CI, 0.28–1.06).61 A more recent analysis of data from this cohort found a strong association between the dose of oral CEE and stroke, with RRs of 0.93, 1.54, and 1.62 for CEE doses of 0.3, 0.625, and 1.25 mg/d, respectively (P < .001 for trend).62
Coronary Heart Disease
Although results from the EPT arm of the WHI indicated that standard-dose therapy may increase the risk of CHD in older postmenopausal women (Table 1),2,64 subsequent analyses of women closer to the menopausal transition in the EPT arm4,16,64 and findings from the ET arm (Table 2)65 suggest a possible cardioprotective effect of HT in women who initiate treatment near the time of the menopause.4,16
A recent analysis of the impact of ET on coronary artery calcification reported that women aged 50 to 59 years at randomization in the WHI who received ET had significantly lower coronary-artery calcium scores after approximately 8 years of treatment compared with those who received placebo (83.1 vs 123.1; P = .02).66 Moreover, ET use was associated with a significantly lower risk of even mild degrees of coronary-artery calcification (score <10 vs >10; odds ratio, 0.74; P = .04), and the effect was stronger in women with at least 80% adherence (odds ratio, 0.55; P < .001). These data indicate that ET use may be related to a reduced coronary plaque burden and a lower prevalence of subclinical atherosclerotic disease.
Although there are minimal data about the impact of low-dose HT on CHD events, existing evidence indicates that lower doses in younger menopausal women do not adversely affect CHD risk.16,67 An analysis of data from the Nurses’ Health Study reported a nonsignificant reduction in CHD risk with lower estrogen doses (RR for CEE 0.3 mg/day, 0.74; 95% CI, 0.52–1.06).16
Lipid Profile
Despite the somewhat controversial impact of HT on CHD, the beneficial effects of standard-dose and low-dose HT on the lipid profile are well established. Typically, estrogen decreases serum levels of total cholesterol and low-density lipoprotein cholesterol and increases high-density lipoprotein cholesterol.42 Because most of this effect is the result of first-pass metabolism, transdermal estrogen generates smaller changes.68 However, oral estrogen increases triglyceride levels, and the addition of a progestin can attenuate some of estrogen's beneficial effects.42 The clinical relevance of these lipid changes has been challenged by the WHI finding that HT use did not provide cardioprotection in the overall study population.2,3,64,65
In general, lipid changes are greater with standard-dose versus low-dose estrogen.69 Nevertheless, even low doses of estradiol delivered by vaginal ring for the purpose of treating vaginal atrophy have been found to provide significant improvements in lipid levels, presumably mediated through modest increases in serum estrone sulfate and estradiol levels.70 Progestin potency may also play a role: recent evidence suggests that the beneficial effects of EPT on lipid levels are similar to those observed with estrogen alone when norgestimate, a progestin with low androgenicity, is administered intermittently in women with elevated lipids at baseline.71 Additional research is needed to determine how lower HT doses affect cardiovascular outcomes.
Talking to Patients about Lower-Dose HT
When counseling patients about lower-dose HT, it is important to understand their knowledge, concerns, and preferences regarding HT. Integrating that perspective with a clinical understanding of each woman's personal health status sets the stage for a tailored discussion of the benefits and risks of HT as they relate to that woman. The discussion should cover the relative effects of current options, including the possible advantages and risks of lower-dose therapy. Education about low-dose options should focus on available information about efficacy, safety, and tolerability.
As in any counseling encounter, clinicians should encourage patients to discuss their questions and main concerns without interruption. Misperceptions regarding perceived risks of HT or benefits should be clarified, preferably using nontechnical language and examples. Giving patients the opportunity to speak will enhance patient satisfaction and the efficacy of the consultation. Providing clear and concise patient education materials can also be quite helpful. Because the risks of major adverse health events associated with HT for a healthy, early postmenopausal woman are very small (<2 per 1000 women/year),2 decisions about treatment should be guided primarily by the patient's individual preferences and values.
Implications and Conclusions
Current guidelines recommend that HT should be used at the lowest effective dose consistent with the treatment goals of the individual. Observational data suggest that the use of lower HT doses is not associated with a significant increase in risk of major adverse events; however, there are minimal data from clinical trials on the long-term safety of these preparations. Large, randomized, controlled trials evaluating the long-term efficacy and safety of low-dose HT are urgently needed.
In keeping with the general clinical precept of using the lowest effective dose, lower-dose HT formulations should be considered for the initial management of menopausal symptoms. Patients newly initiated on HT should be encouraged to promptly communicate symptoms and concerns. Dosage or regimen changes should be considered if treatment goals are not met after a suitable interval, typically 1 to 3 months. The use of lower HT doses is supported by evidence demonstrating that they effectively relieve vasomotor symptoms, treat vaginal atrophy, prevent bone loss, provide adequate endometrial protection, and are better tolerated than standard doses.
Acknowledgments
The author wishes to thank Nicole Cooper (DesignWrite LLC) for writing and editorial assistance.
Notes
This article was externally peer reviewed.
Funding: Funding to support the preparation of this manuscript was provided, through an unrestricted grant, by Novo Nordisk, Inc.
Conflict of interest: Dr. Langer has received research support from GlaxoSmithKline and has served as a consultant and expert witness for Wyeth, Inc.
- Received for publication June 25, 2008.
- Revision received March 3, 2009.
- Accepted for publication March 9, 2009.