Abstract
Purpose: Multiple trials in the past have shown conflicting results of whether cinnamon lowers glucose or hemoglobin A1C (HbA1C). The purpose of this study was to determine whether cinnamon lowers HbA1C in patients with type 2 diabetes. I performed a randomized, controlled trial to evaluate whether daily cinnamon plus usual care versus usual care alone lowers HbA1c.
Methods: I randomized 109 type 2 diabetics (HbA1C >7.0) from 3 primary care clinics caring for pediatric, adult, and geriatric patients at a United States military base. Participants were randomly allocated to either usual care with management changes by their primary care physician or usual care with management changes plus cinnamon capsules, 1g daily for 90 days. HbA1c was drawn at baseline and 90 days and compared with intention-to-treat analysis. This study was approved by an institutional review board.
Results: Cinnamon lowered HbA1C 0.83% (95% CI, 0.46–1.20) compared with usual care alone lowering HbA1C 0.37% (95% CI, 0.15–0.59).
Conclusions: Taking cinnamon could be useful for lowering serum HbA1C in type 2 diabetics with HbA1C >7.0 in addition to usual care.
As the worldwide incidence of diabetes increases, the search for dietary adjuncts to treat this life-altering disease has become far ranging. Cinnamon is purported to be a natural insulin sensitizer, with adverse events of perioral dermatitis and stomatitis reported uncommonly with high intake.1 Both in vitro and in vivo animal studies have shown that cinnamon is an insulin sensitizer.2,3 Kim et al3 showed that intestinal glucosidase activity in rats was increased by cinnamon. Polyphenols within cinnamon have been identified as upregulators of mouse adipocyte insulin receptors.4 Peng et al5 found that polyphenols from cinnamon inhibit the formation of advanced glycation end products in bovine serum albumin.
To date, 5 randomized trials studying cinnamon in humans with type 2 diabetes have been published, with conflicting results.6–10 The results of these studies are mixed, and it is unclear whether any of them were conducted among primary care populations. Two of these studies showed a possible effect of cinnamon on fasting serum glucose, but they did not examine hemoglobin A1C (HbA1C) levels. One study showed no effect on plasma glucose. Two studies—one each done with patients with type 1 and type 2 diabetes—showed no effect of cinnamon on HbA1C (Table 1). A meta-analysis of these studies in divergent populations measuring different parameters showed no effect of cinnamon on HbA1C, glucose, or lipids.11
Summary of Current Randomized Controlled Trials Evaluating Cinnamon Use in Diabetic Patients
This trial strove to replicate the conditions found in primary care, where patients often have medication changes, comorbid conditions, and dietary changes. As such, it was not an efficacy trial with every variable controlled. It attempted to determine whether the intervention of taking cinnamon lowers HbA1C. Effectiveness trials such as this are critical in determining if the interventions that we recommend to patients are effective in the practical world in which patients live. I sought to determine whether add-on treatment with 1 g daily of cinnamon over a 3 month period lowers HbA1C in a population of poorly controlled type 2 diabetics.
Methods
Patients were recruited from the population served by the 96th Medical Group, Eglin Air Force Base, Florida, and were recruited through phone calls from volunteers to known diabetics. Patients were eligible if they were included in the Population Health database as patients with diabetes (International Classification of Diseases, 9th revision, code for diabetes) and had a HbA1c of ≥7.0% on a laboratory blood draw during the last 6 months. Pregnancy, age <18 years, and allergy to cinnamon were exclusion criteria. There was no upper age limit. Recruitment ran from March through May, 2007, and follow-up ended in August 2007.
Recruitment
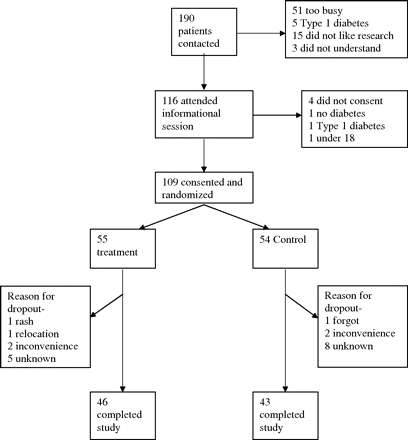
Patients were contacted by volunteers and invited to attend an informational session about the study. One hundred ninety patients were contacted and 116 agreed to attend informational sessions. They were conducted on a rolling basis, with several sessions a week at various times, and the principal investigator or an assistant presented details about the study, distributed and explained informed consent documents, and answered questions. One hundred nine patients agreed to participate and signed informed consent documents at the information sessions (Figure 1). No compensation was offered to volunteers.
Randomization diagram with reasons for exclusion and complete accounting of patients.
Standard demographic data and information regarding the number of diabetes medications, insulin use, and cinnamon intake were collected via an 11-question survey developed by the investigator and tested for clarity on 5 diabetic patients before the study (Table 2). Responses regarding medication use were confirmed in the medical record. Participants were queried about current cinnamon intake (normal, no addition to diet; high, supplemented to diet in any amount or type, ie, capsules, powder, syrup). Participants were asked to continue their pretrial level of cinnamon intake.
Baseline Characteristics of Treatment and Control Groups
Randomization
We randomized patients by blocking (a technique designed to ensure that the treatment and control groups are of similar size) in groups of 10. Marked papers were prepared by a person not otherwise involved with the research team; half of each block of 10 was marked “treatment” and the other half was marked “control.” These were pulled from a box at the time of consent, so allocation was concealed until that time. Neither participants nor investigators were blinded, but the laboratory (true outcome assessor) was blinded to group allocation.
After randomization, all patients were instructed to follow up for their diabetes care as they normally would during the subsequent 90 days. All patients were allowed to adjust their usual medications and doses as recommended by their physician. Each patient had a serum, nonfasting HbA1c drawn on day 0 and again at day 90 to 95. Those patients randomized to take cinnamon received 180 capsules (500 mg each) of Cinnamomum cassia (Puritan's Pride, Oakdale, NY) from the 96th Medical Group pharmacy at the time of randomization. They were instructed to take 2 capsules daily with food in addition to their normal medications. Those randomized to usual care were instructed to take their normal medicines and follow up with their physician as per their normal schedule. The investigator contacted participants by phone and letter at 80 to 88 days after randomization to remind them to have their blood drawn. In addition, information regarding medication changes and cinnamon compliance was collected at this time. All participants had laboratory blood draws done at the same accredited laboratory using the same assay (G7 High Performance Liquid Chromatography Analyzer, Tosoh, Tokyo, Japan).
To determine a power of 0.80, we calculated a sample size of 126, with α = 0.05 (SD, 1). The effect size considered to be clinically significant was a 0.5% lowering of HbA1C.
We analyzed the primary outcome by an unpaired 2-sample t test (mean difference between baseline and final HbA1C). The data sets were normally distributed. An intention-to-treat analysis was performed. Missing data because of withdrawals or noncompliance were dealt with by carrying forward the last known value for HbA1C.
The Institutional Review Board of the 59th Medical Wing, Wilford Hall Medical Center, approved this trial, and the voluntary fully informed consent of the patients used in this research was obtained as required by Title 32 Code of Federal Regulations 219 and Air Force Instruction 40-402.
Results
Baseline characteristics did not differ between the study population groups (Table 2). One hundred nine patients completed the initial visit, where 55 were randomized to the cinnamon (treatment) group and 54 were randomized to the control group. Of these 109, 89 completed the study, giving an 82% completion rate (Figure 1). Ninety-one percent of the patients (42 of 46) completing the treatment arm of the trial reported taking >75% of their cinnamon capsules.
After intention-to-treat analysis of 109 initial patients using the carry-forward method, cinnamon lowered HbA1C 0.83% (95% CI, 0.46–1.20) compared with usual care alone lowering HbA1C 0.37% (95% CI, 0.15–0.59; Table 3). Medication use—a proxy for usual care—did not change significantly in either group during the intervention. Hierarchical analysis of the primary physician being “faculty” versus “resident” was 0; thus, experience level of the primary physician had no impact on HbA1C. Similar results were obtained when analysis was restricted to only the 89 patients who completed the study.
Effect of Cnnamon on Hemoglobin A1C (HbA1C) in Type 2 Diabetics
One patient in the treatment group reported developing a rash that resolved after discontinuing cinnamon. No further adverse effects were identified.
Discussion
This study shows that, over 90 days, supplementation with 1 g of daily cinnamon lowers HbA1C by 0.83% (95% CI, 0.46–1.20) in patients with poorly controlled diabetes.
Strengths and Weaknesses of This Study
This trial was strengthened because it was an effectiveness trial that described community clinical practice and it measured outcomes that are important to diabetes care providers. Trials of efficacy are appropriate to identify whether an intervention works, but without effectiveness data in the types of patients who will receive the intervention it is difficult for clinicians to determine whether to incorporate it into their practice.
We used standard, off-the-shelf cinnamon capsules that patients would find at their local stores or on the Internet. We did not test these capsules for purity because there is no regulation of cinnamon as a food supplement and we wanted to mimic cinnamon that patients would buy on their own.
The primary reason that no placebo was used in this study was the strong taste of cinnamon capsules. This precluded any blinding of patients because they would know within minutes whether they had taken the treatment or not. This lack of placebo made it difficult to prove beyond any doubt that the cinnamon itself versus the daily reminder to take the cinnamon provided was the cause of the lowered HbA1C.
Recruitment stopped before reaching the predicted sample size because of the unplanned relocation of the investigator. A statistically significant benefit is still seen with fewer numbers.
Strengths and Weaknesses in Relation to Other Trials
Previous trials have shown conflicted results about the efficacy of cinnamon to treat diabetes. These trials have a variety of shortcomings: they generally studied well-controlled patients with type 2 diabetes7,8,10; some only measured fasting glucose6; some measured HbA1C after as little as 30 days’ time7; some had narrow age, sex, or racial limits to their study populations6–9; all excluded insulin use and comorbid conditions; and none allowed medication changes. Only one included a power analysis.10 Although meta-analysis of these trials11 is interesting, the diverse nature of the trials makes it very difficult to draw useful conclusions from a combined analysis.
There are several reasons why this current trial may have shown benefit where earlier trials did not. First, this is the largest randomized cinnamon trial to date in type 2 diabetics. Second, we were more likely to see a treatment effect because we studied only patients with poorly controlled type 2 diabetes. Third, it is possible that the inclusion of patients using insulin and those with comorbid conditions potentiated the effect of cinnamon, but this is unlikely because the groups were similar. Finally, diabetics in the United States may have different characteristics than those in other countries.
The Meaning of This Study
This study gives diabetes care providers and diabetic patients an easily accessible, likely safe, and cheap alternative to help treat type 2 diabetes. According to the United Kingdom Prospective Diabetes Study,12 a drop of HbA1C from 7.9% to 7% lowers the risk of macrovascular disease 16%, retinopathy 17% to 21%, and nephropathy 24% to 33%; thus, a 0.83% drop in HbA1C levels in patients might be expected to yield similar reductions in morbidity.
Unanswered Questions and Future Research
Whether there is synergy between cinnamon and prescription medications (including insulin) is a topic for further research. Some have postulated that lipids are lowered by the use of cinnamon. Because one of the proposed mechanisms of cinnamon is increased insulin sensitivity, the treatment of patients with the metabolic syndrome with adjunct cinnamon may yield weight loss, improved lipid profiles, and better glucose tolerance. Both in vitro and in vivo animal studies have shown that cinnamon is an insulin sensitizer.2 Several compounds within cinnamon have been identified as possible sources of this sensitization process.
Conclusion
One gram of daily adjunct cinnamon in addition to usual care seems to lower HbA1C in patients with poorly controlled type 2 diabetes. Cinnamon supplementation is likely to be safe and may be offered to patients with HbA1C >7.0% as a potential means to lower HbA1C.
Acknowledgments
The author would like to acknowledge the significant contributions of Charles and Eileen Arpke and Lt. Col. Karen Weis to patient enrollment.
Notes
This article was externally peer reviewed.
Funding: Funding for this study was provided by the United States Air Force Surgeon General's Office for Population Health Research.
Prior presentation: This work was presented at the Society of Teachers of Family Medicine Annual Meeting, Baltimore, MD (April 30 through May 4, 2008).
Conflict of interest: none declared.
Disclaimer: The opinions and assertions contained herein are the private views of the author and not to be construed as official or as reflecting the views of the US Air Force Medical Service or the US Air Force at large.
Trial registration: ClinicalTrials.gov identifier: NCT00445354.
- Received for publication May 11, 2008.
- Revision received May 2, 2009.
- Accepted for publication May 7, 2009.