Abstract
Purpose: Hyperuricemia is associated with increased cardiovascular risk. Because patients with asymptomatic hyperuricemia (AH) experience no immediate discomfort and there are possible side effects of urate-lowering drugs, treatment for AH is controversial. We aimed to perform a network meta-analysis (NMA) to investigate the effects of different urate-lowering therapies (ULTs) on serum uric acid level, renal function, blood pressure (BP), and safety in AH patients.
Methods: This NMA focused on AH patients. The intervention group (patients receiving urate-lowering drugs) was compared with others using other types of drugs, placebo, or usual care. We undertook a NMA under the frequentist framework by R.
Results: Thirteen eligible trials were identified. The interventions included allopurinol, febuxostat, and benzbromarone, which are not approved in the United States. Benzbromarone and allopurinol had the best efficacy on lowering serum uric acid level in short-term and long-term follow-up (mean difference [MD] = −3.05; 95% CI, −5.19 to −0.91 vs MD = −3.17; 95% CI, −5.19 to −1.15). Patients using allopurinol had significantly higher eGFR than using placebo in both short-term and long-term follow-up (MD = 3.07; 95% CI, 0.18 to 5.95 vs MD = 4.10; 95% CI, 2.66 to 5.54). No difference in BP was found between groups, except for febuxostat to diastolic BP after long-term treatment (MD = −1.47; 95% CI, −2.91 to −0.04). No statistically increased odds of safety events were found with the use of ULT.
Conclusions: Our result showed that in AH patients, allopurinol has a renoprotective effect. Febuxostat has a significant impact in lowering diastolic BP. ULT does not result in a higher risk of safety events.
- Asymptomatic Hyperuricemia
- Blood Pressure
- Disease Management
- Family Medicine
- Network Meta-Analysis
- Serum Uric Acid
- Systematic Review
- Renal Function
Introduction
Vascular endothelium, a monolayer of endothelial cells, controls vascular tone and maintains vascular homeostasis, allowing it to maintain normal physiologic mechanisms.1 Endothelial dysfunction means endothelial cells lose their normal function and is found to be associated with hypertension and chronic kidney disease (CKD).2,3 Hyperuricemia is 1 of its causes, and urate-lowering therapy (ULT) is proved to improve endothelial function.4⇓⇓–7 Therefore, many trials investigated whether patients under ULT attained better blood pressure (BP) control and renal function.8⇓–10 ULT is commonly prescribed for patients if any symptom or sign of hyperuricemia develops.
However, more than half of hyperuricemic individuals remain asymptomatic.11 Asymptomatic hyperuricemia (AH) is defined as hyperuricemic patients without either symptoms or signs of gout, tophi, hyperuricemic nephropathy, or uric acid nephrolithiasis.12 Because there are possible side effects of urate-lowering drugs, treatment for AH is controversial.13,14 Urate-lowering drugs include xanthine oxidase inhibitors, such as allopurinol and febuxostat, and uricosuric agents, such as benzbromarone and probenecid. Severe skin reaction, higher cardiovascular (CV) risk or impaired liver function related to those drugs have been reported.15⇓⇓⇓–19 Benzbromarone was, therefore, withdrawn from the market in 2003 and has never been approved in the United States due to its reports of hepatotoxicity. 20,21 Japanese guidelines for managing hyperuricemia and gout recommend initiating ULT for AH when serum urate levels increase to > 8.0 mg/dL.22 However, this approach is not recommended in the United States and Europe owing to the side effects of these drugs.14
Xanthine oxidase inhibitors are thought to have the potency to decrease oxidative stress causing endothelial dysfunction.10,23 The metabolite of allopurinol is excreted predominantly by the kidney, and febuxostat is believed to be safe for patients with CKD owing to its hepatic elimination.24 The comparative effects of these drugs have not been investigated.
Network meta-analysis (NMA) is, therefore, a useful tool because it can use both direct and indirect evidence to compare the effects of all ULT. In contrast, previous meta-analyses either considered ULT as a single group or compared each drug to the control separately. Therefore, we conducted a systematic review and NMA to investigate the effects of different urate-lowering drugs on serum uric acid level, renal function, and BP in patients with AH. We would also investigate the safety of those treatments to attain a balanced consideration for AH patients.
Methods
We conducted a systematic review and NMA of randomized controlled trials on patients with AH. The intervention group (patients receiving urate-lowering drugs) was compared with groups of other types of urate-lowering drugs, placebo, or usual care. The outcomes were serum uric acid level, renal function, BP, and adverse events. We registered our systematic review on PROSPERO website. This NMA followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) extension guideline, which incorporated NMA for health care interventions and was registered in PROSPERO (registration number: CRD42021256528).
Literature Search
Two investigators (YYT and CPT) independently searched PubMed and Embase from their inception through October 8, 2020. We had also searched at ClinicalTrials. gov and hand-searched reference lists of relevant publications. The population of included trials was AH patients. Given that there are some controversies over the definition of hyperuricemia, we respected authors' definition of hyperuricemia in each study.12 If “asymptomatic” was not used to describe its population, a trial was still considered eligible if it enrolled patients without a history of gout or other related symptoms. Chronic hyperuricemic nephropathy is usually asymptomatic and is not easy to diagnose. If a trial described its patients as AH and with CKD, this was interpreted as that CKD in those patients was not caused by their hyperuricemia. Therefore, those studies would still be included. We used the keywords “hyperuricemia,” “asymptomatic,” “urate-lowering therapy,” and classification or name of the drugs for searching. The search details are shown in Appendix 1. The bibliographies of recent review articles and previous meta-analyses were also manually searched for relevant studies.
Study Outcome
The primary outcomes were serum uric acid level, measured in units of mg/dL, renal function, assessed by estimated glomerular filtration rate (eGFR), and BP, measured in units of mmH,g and divided to systolic and diastolic BP. The eGFR was calculated with 1 of the following methods: Cockcroft-Gault formula, the 4-variable modification of diet in renal disease study equation, or CKD epidemiology collaboration equation. The secondary outcome was adverse events, including the occurrence of impaired liver function, gastrointestinal event, CV event, skin reaction, and musculoskeletal event in patients within the trials identified by our search strategy.
Study Selection
All titles and abstracts retrieved from the literature search were screened by 2 reviewers to determine the eligibility of a study. We included clinical trials where patients were randomly allocated to receive different treatments or placebo/usual care groups. We excluded conference proceedings without full text, nonrandomized controlled trials, the intervention group not receiving approved medicine, and studies not specific to asymptomatic adults.
Data Extraction
The outcomes were extracted independently from the included studies by 2 investigators mentioned above. For the primary outcomes, we evaluated the treatment effect by dividing the duration of treatment into short-term (≤ 6 months) and long-term follow-up (> 6 months). We assumed that it takes at least 6 months for a drug to show a robust effect, so we used 6-month to separate the short and long-term effects.
For the secondary outcomes, we analyzed events of impaired liver function, gastrointestinal events, CV events, skin reaction, and musculoskeletal events. Details are shown in Appendix 2.
Quality Assessment of Methods
We used Cochrane Risk of Bias Tool to assess the quality and risk of bias for the included studies (Appendix 3). We defined the risk of bias as adequate, unclear, or inadequate for assessing 6 aspects of the trials: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. The assessment was conducted by 2 independent reviewers, with a third consulted for resolution of any disagreements.
Statistical Analysis
We used “meta,” “netmeta” and “dmetar” packages for the free statistical software R (version 4.0.3, Vienna, Austria) to undertake a frequentist pairwise meta-analysis and NMA.
NMA uses both direct and indirect evidence to compare multiple interventions within a statistical model. If 2 interventions have never been compared head-to-head, but both have been compared with a common comparator (such as placebo), an indirect comparison can be evaluated via the common comparator. 25 An estimate of mean difference (MD) in treatment effect between 2 interventions is a weighted average of direct and indirect comparisons, with confidence intervals (CI).
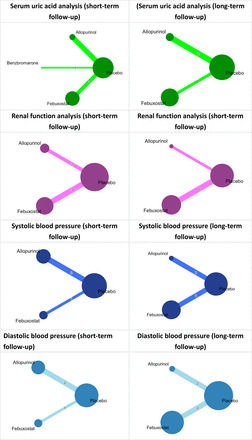
For each primary outcome, we created network plot which shows the overall structure of comparisons in the NMA. The size of the circles is proportional to the number of patients randomized to each intervention, and the width of the edges is proportional to the number of studies making each comparison.
We had also performed pairwise meta-analyses of all head-to-head comparisons to evaluate the heterogeneity within each comparison.25
For continuous outcomes, such as serum uric acid level, eGFR, and BP, we estimated the difference in mean changes between the treatment and control groups. If a trial did not report such a result, we would calculate the difference in the follow-up measurements between 2 groups at a specific time point. We used the recommended methods by the Cochrane Handbook to impute missing values.26 League tables were created to summarize the results of pairwise comparisons from NMA. If a trial reported 2 or more results within the period, we used data of the shortest follow-up for short-term analysis and the longest follow-up for long-term analysis to distinguish the short-term and long-term effects better. For dichotomous outcomes, such as safety outcomes, we used the Peto odds ratio model because the event numbers were small or even zero in some studies.26 The study effect sizes were then synthesized using a random-effects NMA model.
To rank the treatments for each outcome, we used P-score, which measures how likely a treatment is better than the other competing treatments. P-scores are derived from the P values of pairwise comparisons for a treatment is compared with the other treatments in the network. P-scores reflect the differences between the point estimates of treatment effects but also take the precision into account. The range of P-scores is from 0 to 1, and a large P-score (eg, >0.90) suggests a high certainty of a treatment being more effective or safer than others. 27 However, P-scores are descriptive, and a large difference between 2 P-scores does not necessarily mean the difference between the 2 treatments is statistically significant. There is no formal method to test the difference in P-scores either.
If both direct and indirect evidence is available for a comparison between 2 treatments, we use the design-by-treatment interaction model and node-splitting model to evaluate the consistency between direct and indirect evidence. We evaluated the assumption of transitivity for indirect comparisons by examining the distribution of confounding variables, such as baseline kidney function, or undertook subgroup analyses if the number of included studies is sufficient to conduct such analyses.
Results
Our literature search identified 777 potentially eligible studies. Thirteen randomized controlled trials were finally included in our systematic review, totaling 2842 people.28⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–40 Figure 1 shows the study selection process in detail. Table 1 outlines the basic characteristics of the included studies. The intervention included allopurinol, benzbromarone, and febuxostat. The results of a pairwise meta-analysis on direct comparisons are shown in Appendix 6. Most comparisons show no substantial heterogeneity between studies.
Flowchart of the process to identify eligible studies with reasons for inclusion or exclusion.
Overview of Included Studies
Primary Outcome
Short-Term Urate-Lowering Effect
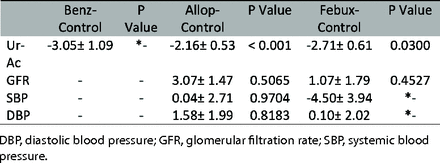
Eight studies were included in the analysis of the urate-lowering effect for short-term (≤ 6 months) follow-up.29,30,32,33,35,37,39,40 The network plot and results of our NMA are summarized in Appendix 4 and Table 2. Patients used allopurinol, benzbromarone and febuxostat showed significantly lower serum uric acid level compared with placebo (MD = −2.16 mg/dL; 95% CI, 3.2 to −1.13 vs MD = −3.05 mg/dL; 95% CI, −5.19 to −0.91 vs MD = −2.71 mg/dL; 95% CI, −3.9 to −1.52), but there were no significant differences between drugs. Benzbromarone had the highest P-score of being ranked first for urate-lowering efficacy (Table 3).
League Table of Random-Effects Network Meta-Analysis for Effect of Urate-Lowering Therapy*
P-Score of Different Rankings of Each Treatment Strategy
Long-Term Urate-Lowering Effect
Three studies reported a long-term (> 6 months) urate-lowering effect.28,31,40 The network plot and results of our NMA are summarized in Appendix 4 and Table 2. Patients using allopurinol had significantly lower serum uric acid level compared with placebo (MD = −3.17 mg/dL; 95% CI, −5.19 to −1.15). Patients using febuxostat had lower blood uric acid levels (but not significantly different) compared with placebo. The serum uric acid level showed no significant difference between drugs. Allopurinol had the highest P-score of being ranked first for better urate-lowering efficacy (Table 3).
Renal Function: Short-Term Follow-up
Five studies were included in this analysis.30,32,34,37,39 The intervention included allopurinol group and febuxostat group, and the network plot and results of our NMA are summarized in Appendix 4 and Table 2. Patients used allopurinol had significantly higher eGFR compared with placebo (MD = 3.07 mL/min/1.73m2; 95% CI, 0.18 to 5.95). Patients who used febuxostat had a higher eGFR (but not significantly different) compared with placebo. Besides, allopurinol group also had higher eGFR compared with febuxostat group, but no statistical significance was found. Allopurinol had the highest P-score of being ranked first for better renal function (Table 3).
Renal Function: Long-Term Follow-up
Three studies were included in this analysis.31,36,40 The intervention included allopurinol group and febuxostat group. Appendix 4 and Table 2 showed the network plot and results of our NMA. Patients used allopurinol had significantly higher eGFR than using febuxostat or placebo (MD = 3.70 mL/min/1.73m2; 95% CI, 1.94 to 5.46 vs MD = 4.10 mL/min/1.73m2; 95% CI, 2.66 to 5.54). Patients used febuxostat had higher eGFR than using placebo but without statistical significance. Allopurinol had the highest P-score (Table 3).
Blood Pressure: Short-Term Follow-up
Three eligible studies were included, and the network plot and results of our NMA for systolic/diastolic BP are summarized in Appendix 4 and Table 2.30,34,35 No significant difference in systolic/diastolic BP between groups was found. P-score was summarized in Table 3.
Blood Pressure: Long-Term Follow-up
Four studies were included, and Appendix 4 and Table 2 showed the network plot and results of our NMA.28,31,36,40 No significant difference of systolic/diastolic BP was found between groups, except patients in febuxostat group had 1.47 mmHg statistically lower diastolic BP than patients in placebo group (MD = −1.47 mmHg; 95% CI, −2.91 to −0.04). P-score was summarized in Table 3.
Secondary Outcome: Adverse Events
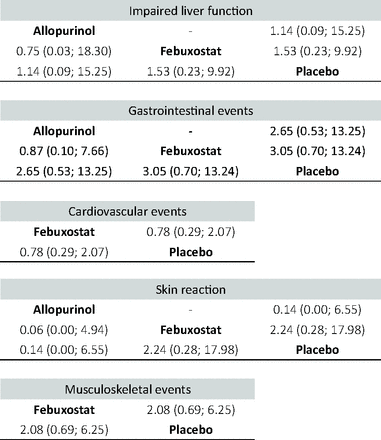
Six trials, 1269 patients, were included in the analysis of impaired liver function.29,31,34⇓–36,40 Six trials, 986 patients, were included in the analysis of gastrointestinal events.31,32,34,35,37,40 Five trials, 1195 patients, were included in the analysis of cardiovascular event.32,34,36,37,40 Four trials, 1102 patients, were included in the analysis of musculoskeletal events.34,36,37,40 Three trials, 1009 patients, were included in the analysis of skin reaction 35,36,40 Compared with placebo via NMA, ULT did not significantly increase the odds of any secondary outcome (Appendix 5).
As no treatment groups formed a loop in any outcomes, we could not evaluate inconsistency between direct and indirect evidence. No subgroup analysis was undertaken because the number of the low number of included studies. Baseline eGFR of patients showed quite a wide variation across the included trials, but the assumption of transitivity was not considered seriously violated due to the hepatic metabolism of febuxostat, and both similar and typical dose was used in most trials of allopurinol. 28,30,31,33,35,39
Discussion
Our NMA showed that benzbromarone and allopurinol have the best efficacy on lowering serum uric acid levels in short-term and long-term follow-up within AH patients. Patients using allopurinol have better eGFR than using placebo. ULT seems to have no significant effect on BP, except for febuxostat on diastolic BP after long-term treatment. ULT does not significantly increase the risk of safety outcomes. Asymptomatic patients are often neglected for treatment, and our results provide much-needed evidence for treating those patients to attain better renal function.
Uric Acid
Previous meta-analysis or NMA included patients who were mostly symptomatic, so the doses of their drugs were relatively larger than those we recruited. Li et al reported a NMA for comparing efficacy of ULT in patients with or without gout.41 Their results showed benzbromarone (100 to 200 mg/day) had better urate-lowering effect than allopurinol (100 to 600 mg/day), and allopurinol (100 to 600 mg/day) had better urate-lowering effect than febuxostat (20 mg/day). In our NMA, only 1 trial reported the result of benzbromarone with a dose of 50 mg/day, but we still found a similarly strong effect of benzbromarone in the short-term follow-up. However, no trial on benzbromarone reported results with more than 6 months of follow-up, so its long-term efficacy is uncertain. Our result showed that allopurinol (starting from 100 mg/day) had better effect on lowering serum uric acid levels than febuxostat (10 to 60 mg/day) in the long term. This result partly agrees with what Li et al found that allopurinol had a better effect than a low dose of febuxostat.41
Nevertheless, the effect on uric acid is related to the dose of drugs. The selection of drugs and their doses also depends on patients' kidney function, responses to the treatment, and other factors.
Renal Function
Meta-analysis by Kanji et al showed patients with CKD using ULT had significantly better eGFR with a mean difference of 3.2 mL/min/1.73 m2 than using placebo.42 Slower eGFR decline rate by 4.1 mL/min/1.73m2 per year compared with control group was found in the study of Su et al43 Those meta-analyses focused on patients with CKD and were not limited to asymptomatic patients. Our NMA included more diverse population, not only patients with CKD, but the result still showed that patients using allopurinol had 3.07/4.1 mL/min/1.73m2 significantly higher eGFR than using placebo in short-term/long-term follow-up. Although the differences are small, they may be of great significance for patients who already have kidney disease. In addition, the results were similar to previous research.42,43
Our result showed that febuxostat yielded a nonsignificant increase in eGFR compared with placebo. This was similar to a meta-analysis by Li et al which included symptomatic and asymptomatic CKD patients.44 As only 3 trials were included in their meta-analysis and 5 trials included in ours; these nonsignificant benefits may become significant if the number of subjects increases.
We did not find any trial of uricosuric agents reporting renal function of asymptomatic patients, so we cannot distinguish the possibly different effect between xanthin oxidase inhibitors and uricosuric agents.
Blood Pressure
The meta-analysis by Qu et al found allopurinol found a greater reduction in systolic BP and diastolic BP.45 They included patients with hyperuricemia with or without symptoms, so the dose of allopurinol (100 mg/day to 900 mg/day) was relatively larger than our studies. This may explain why allopurinol showed smaller effects on BP in our analysis. We found a decreasing trend of systolic BP under treatment of allopurinol and febuxostat in the long-term follow-up, but the effect of ULT on BP needs more research.
Safety
White et al found that in patients with gout and major CV coexisting conditions, using febuxostat showed higher all-cause mortality and CV mortality than using allopurinol in a median of 32 months in 6190 patients.17 Five trials, totaling 1195 patients, were recruited in our NMA reporting CV events.32,34,36,37,40 The result showed patients using febuxostat did not have a higher risk than those using placebo. However, no allopurinol-related trial was included in our analysis, so we could not compare the effects of these 2 drugs on CV events. The longest follow-up period in these trials was 27 months, but CV events may require more time and more patients to observe.
Allopurinol is frequently associated with Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN).15 Three trials in our NMA, totaling 1009 patients, reported skin reaction and did not show a higher risk of skin reaction in patients using allopurinal.35,36,40 Previous reports showed that the incidence rates of SJS/TEN range from 1.4 to 12.7 cases per million person-years.46,47 Therefore, such serious skin reaction is rare if the patient number is not large enough.
Strengths and Limitations
The strength of our NMA was that we focused on patients with AH and compared the efficacy of individual drugs. We also divided the treatment duration into short-term and long-term. However, this study has some limitations. First, only 3 drugs, allopurinol, febuxostat, and benzbromarone, were included in our analyses, while probenecid, lesinurad, and other urate-lowering drugs were not because these drugs had not been studied among AH patients. Second, no head-to-head trials that compared allopurinol and febuxostat were included in our analysis. Although it is the advantage of NMA that an indirect comparison can still be undertaken for these 2 treatments as both have been compared with placebo, we cannot verify the results because we do not have data from a direct comparison.48 Thirdly, the number of the included studies was too few to undertake subgroup analysis. For instance, only 1 trial focusing on CKD population was included in the analysis of long-term renal function, so we could not compare the efficacy of those drugs on renal function among CKD patients. In our NMA, the included trials recruited patients of different comorbidities. However, considering the kidney plays a major role in uric acid homeostasis, we felt that renal function was the most important factor, and we noted that the average eGFR of each trial in our analysis was different. Febuxostat undergoes hepatic metabolism, and its dose adjustment and effects are less affected by patients' renal function.49 Trials on allopurinol used similar doses, 200 to 300 mg/day, 50 and this range of dose is considered suitable for CKD patients included in our NMA.51 Although the heterogeneous populations should be considered in the interpretation of our results, we felt that the assumption of transitivity was not seriously violated. Fourthly, our results showed Allopurinol has a renoprotective effect, and this finding seems quite robust in Asian population as our results were mainly derived from Asian studies. More randomized controlled trials from non-Asian countries are required to verify the protective effect.
Conclusions
Our result showed that in AH patients, benzbromarone and allopurinol have the best urate-lowering effect in the short-term and long-term follow-up. Allopurinol has a significant renoprotective effect. Febuxostat has a significant effect on lowering diastolic BP in long-term follow-up. ULT does not result in a higher risk of impaired liver function, gastrointestinal event, CV event, skin reaction, and musculoskeletal event. According to the above results, patients with AH may be treated with ULT to benefit from renal protection, and the use of allopurinol should be considered a priority.
Appendices
Appendix 1. Literature search strategy
Appendix 2. Data extraction from included trials- Details of secondary outcomes
Appendix 3. Summary of the risks of bias in every included trial
Appendix 4. Network plot for effect of urate lowering therapy
Appendix 5. League table of the network meta-analysis comparing the events of secondary outcomes of all drugs
Appendix 6. Result of pairwise meta-analyses of all directly compared interventions
Literature search strategy
Data extraction from included trials: Details of secondary outcomes
Summary of the risks of bias in every included trial
Network plot for effect of urate-lowering therapy. Each node represents a treatment group, and an edge indicates at least 1 trial comparing the 2 treatments on the ends of the edge. The node size in the network plot is proportional to the number of patients randomized to the treatment group, and the width of an edge is proportional to the number of studies making the pairwise comparison
League table of the network meta-analysis comparing the events of secondary outcomes of all drugs, including odds ratios and 95% confidence intervals
Result of pairwise meta-analyses of all directly compared interventions Appendix 6.1 Results of pairwise meta-analyses of all directly compared interventions of short-term results. P value is obtained from the Cochrane Q test for heterogeneity.
Results of pairwise meta-analyses of all directly compared interventions of long-term results. P value is obtained from the Cochrane Q test for heterogeneity.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/35/1/140.full.
- Received for publication July 3, 2021.
- Revision received September 8, 2021.
- Accepted for publication September 15, 2021.