Abstract
Background: Restless legs syndrome (RLS) is a sensorimotor disorder that can have a considerable negative impact on quality of life and sleep. Management is primarily pharmacological; nonpharmacological options are limited. The objective of the present study was to determine the effect of tension and trauma release exercises on RLS severity compared with discussion group controls.
Methods: Participants satisfied RLS diagnostic criteria, did not have acute mental health conditions, and reported being physically able to complete exercises. Eighteen participants (stratified by age and RLS severity) were randomly allocated with concealment to once-weekly sessions of trauma release exercises (n = 9), exercises to stretch and fatigue lower limb muscles and invoke therapeutic tremors, or control discussion groups (n = 9) for 6 weeks. Outcomes assessed at baseline and each week were International Restless Legs Syndrome Rating Scale scores, global RLS severity ratings (visual analog scale, 0 to 10), global stress ratings (visual analog scale, 0 to 10), Pittsburgh Insomnia Rating Scale scores and Major Depression Inventory scores.
Results: There were no significant between-group differences at baseline except for more severe global RLS scores for controls (P = .003). There were no significant between-group differences at week 6 on any outcome. Significant improvements across time were seen for both groups on all outcomes.
Conclusions: In this exploratory study, tension and trauma release exercises and attending discussion groups were associated with similar outcomes. Participants in both groups improved similarly across time. Future research might establish score stability across a prolonged baseline before commencing intervention.
- Exercise Therapy
- Lower Extremity
- Mental Health
- Quality of Life
- Restless Legs Syndrome
- Visual Analog Scale
Restless legs syndrome (RLS), also known as Willis-Ekbom disease, is a sensorimotor disorder characterized by an uncomfortable urge to move the legs.1 The urge to move is usually accompanied by unpleasant sensations in the legs, which worsen in the evening or night and during rest or inactivity, and are relieved by movement.2 RLS can have considerable negative impact on quality of life and sleep. People with RLS have a higher than typical prevalence of anxiety and/or depression, and approximately 50% to 85% experience troubling insomnia.3 RLS has a prevalence of 5% to 15% in the general population,3 however, is underdiagnosed2 and often inadequately treated.4 It is more common in women5 and prevalence increases with age.6 RLS may be primary (idiopathic), or secondary and associated with conditions such as iron deficiency anemia, pregnancy or end-stage renal disease.2 As secondary RLS is addressed through management of the underlying condition, this study focused on idiopathic RLS. Periodic limb movements in sleep (PLMS) are repetitive movements of the lower extremities during sleep. Although PLMS is not specific to RLS and not an essential diagnostic criterion for RLS, a high PLMS index (number of PLMS per hour of sleep) supports a diagnosis of RLS.2
Medications for RLS can have serious side effects such as augmentation. This worsening of symptoms2 is associated with dopamine agonists7 and levodopa.8,9 Many people with RLS therefore seek alternate nonpharmacological options. However, high-quality guidelines published by Aurora et al10 (AGREE assessment “recommended”11) concluded that there was insufficient evidence for nonpharmacological therapy. A recent review by the authors of eleven randomized trials12⇓⇓⇓⇓⇓⇓⇓⇓⇓–22 concluded that some nonpharmacological interventions including exercise and acupuncture seem beneficial for RLS symptoms; however, few studies were identified and the level of evidence was often not high. Given common unwanted side effects associated with pharmacological management, nonpharmacological treatment options may be valuable for symptom reduction.
Tension and trauma release exercises (TRE) invoke what protagonists refer to as “neurogenic tremors.”23 Although these exercises are promoted in Australia24 and the United States,25 reports of positive effects on RLS outcomes are anecdotal. Berceli and Maria26 hypothesized that involuntary movements and tremors in the body may be part of the body's natural mechanism to relieve tension and restore homeostasis. TRE practitioners suggest that tremors, invoked by activities that fatigue muscles, behave to some extent like clonus. This clonus-like tremor seems to be under voluntary control, continuing until an attempt is made to overcome it. TRE practitioners report that tremors, once established, can increase in magnitude and “spread” to other parts of the body that have not undergone fatiguing exercises. The technique of allowing tremor is the focus of the TRE program. Berceli23 proposed that the exercises release deep chronic tensions in the body. Proposed claims regarding the potential merit of TRE for RLS are built on the unsubstantiated hypothesis that RLS may be the result of chronic physiologic stress to the body. If the exercises release chronic tension, they may affect symptoms.
The primary objective of this exploratory trial was to investigate the effect of TRE on RLS severity compared with discussion group controls. Secondary aims were to compare TRE to control conditions on the outcomes of sleep quality, global stress, and depression. The null hypothesis was that there would be no difference in RLS severity scores for those allocated to intervention or control conditions.
Methods
Trial Design
The study was a randomized controlled trial comparing TRE to a control group for participants with RLS. The intervention involved participation in a weekly exercise group. The control condition involved attending a discussion group on the same days, at the same time, and for the same duration as the exercise group. Measurements of key outcomes were collected before each session for both groups. The study was reported using the CONSORT recommendations.27 Institutional ethics approval was granted (Monash University Human Research Ethics Committee CF14/1931-2014000986). The trial was registered in the Australian New Zealand Clinical Trials Registry, ACTRN12615000011583.
Participants
Participants were included if they satisfied all 4 RLS diagnostic criteria,2 did not have an acute mental health issue, and reported that they would be physically able to complete the exercises. The study took place at Monash University Peninsula Campus–Victoria from March to May 2015.
Recruitment
Participants were recruited using internet advertising on the Trauma Release Exercises Australia Web site, in the Sleep Disorders Australia newsletter, and through advertisements at Monash University and local physiotherapy and general practice clinics. To counter the potential for coercion, only administrative staff in clinics could invite participants to join the study. Interested participants were screened for eligibility by phone. Those who met inclusion criteria were advised that they would be randomly assigned to either immediate or a delayed intervention and informed regarding the nature of the intervention and control conditions, attendance requirements, and program location. They were also advised that if they were not allocated to the intervention group, they would be enrolled in a 6-week exercise program commencing after the 6-week trial. Those who remained interested were enrolled after providing written informed consent. They were asked to continue as far as practicable with their current management.
Interventions
Trauma Release Exercises
The intervention consisted of an initial 3-hour introductory session, and a 1-hour session each week for the following 5 weeks (total 6 weeks). The program was designed to allow repeated reinforcement of the exercises and optimize exercise mastery, so participants could use the exercises in ongoing self management. Exercise instruction was delivered by a certified instructor. Participants were taught to invoke “self-induced therapeutic tremors” using structured progressive exercises. Table 1 summarizes the full procedure which has 2 parts: 1) preparatory, and 2) floor-based exercises. Participants were able to stop self-induced tremors by either straightening their legs to take the hip adductors off load, or consciously holding the limbs still. The process was fully self regulated and participants were educated to maintain comfort and control throughout. Participants could therefore self manage their fatigue and emotional levels. When participants were able to start and stop the self-induced tremors, they were encouraged to practice the exercises and invoke tremors at home. Participants were encouraged to use the floor-based exercises alone as needed when symptoms were acute.
Trauma Release Exercises (TRE) Procedure Summary
Control Group
Participants in the control condition participated in discussion exploring the lived experience of RLS. These sessions involved seated discussion and did not include active exercise. Discussions were led by an experienced facilitator and audio recorders captured the dialog. Session duration was matched to intervention group conditions. In the first week (3-hour session), participants were encouraged to talk about their condition in response to open questions. Discussion focused on participant experiences of the condition, and how they managed symptoms. Issues identified in week 1 were explored during subsequent sessions (5 × 1–hour duration). Qualitative analyses of discussion group data will be reported elsewhere.
Outcome Measures
Primary Outcome Measures
The primary outcome measure for RLS severity was the International Restless Legs Syndrome Rating Scale (IRLS), a 10-item questionnaire developed through expert evaluation of potential items. It has high internal consistency, interexaminer reliability, and test-retest reliability over a 2-to-4-week period,28 and is considered the standard measure of RLS severity.29
Secondary Outcome Measures
RLS has also been associated with insomnia and psychological comorbidities such as depression and anxiety, possibly due to the influence of symptoms such as fatigue, sleep disturbance, and poor concentration.3 Outcome measures for sleep, anxiety, and depression were therefore included. Two visual analog scales (0 to 10) were used, 1 for global RLS severity and 1 for global stress level ratings, where 0 indicated no RLS symptoms or no stress and 10 indicated very severe symptoms. The Major Depression Inventory (MDI) is a self-rated inventory developed to measure depression. The MDI has been reported to have acceptable sensitivity and specificity.30 The following MDI response options were used: all the time (4), most of the time (3), slightly more than half of the time (2), some of the time (1), at no time (0). No clear recommendations for assessing sleep related outcomes in RLS have been proposed.31 The Pittsburgh Insomnia Rating Scale (PIRS) is a 65-item scale designed to assess severity of insomnia.32 Sleep quality in this study was measured using the modified 20-item version (PIRS-20). The 20 items were selected to include daytime and night time items, and excluded the influence of items assessing symptoms of depression and anxiety.33 It focused on sleep and wake symptoms as they occurred in the week before completion of the instrument. The PIRS-20 has been reported to be reliable (Cronbach's α, 0.95,=; test-retest reliability, 0.92).33 Each week during the study, all participants were asked to report whether RLS symptoms were experienced in their arms, legs, or both.
Participants in the intervention group completed a weekly exercise diary to monitor home exercise adherence. Attendance data and adverse events of the intervention and control conditions were collected and recorded.
Participant Demographic Data
Data on participant characteristics were collected for both groups using an online survey 1 to 2 weeks before commencement of the study, and included age, gender, family history of RLS, medications, other therapies, comorbidities, and Human Activity Profile (HAP) scores. The HAP provided 2 measures of baseline physical activity. The Maximum Activity Score (MAS) is the number of the highest energy-expenditure item marked, and the Adjusted Activity Score is the MAS minus the total number of lower scoring items that the person has stopped doing.34 IRLS scores were also collected at this time to stratify participants by severity of RLS during random allocation.
When Outcomes Were Assessed
Baseline measures were taken immediately before commencement of the intervention and control sessions, and included IRLS scores, global RLS scores (0 to 10), global stress scores (0 to 10), PIRS scores and MDI scores (week 0). All outcomes were reassessed immediately before each exercise and discussion group session (weeks 1 to 5) and collected using an online survey if participants were not able to attend. Post-intervention data (week 6) were collected online.
Sample Size
Sample size was determined using power analysis for the outcome of IRLS scores (scale 0 to 40) based on results of similar studies where physical interventions for RLS were compared with control groups.12,18,20 With a sample of 6 in each group the study would be powered (0.8) to detect a difference between means of 9/40 IRLS points when α was set at 0.05 using a 2-tailed t-test assuming a standard deviation of 5.9. Mean changes and variance estimates of this magnitude comparing baseline to postintervention scores are typical of those observed in similar studies.12,18,20 Allowing for 20% attrition we aimed to recruit at least 8 people to each group (total n = 16).
Randomization
Sequence Generation, Concealment, and Allocation
Participants were stratified for RLS severity (IRLS score > 21, severe/very severe and < 20, mild/moderate RLS) and age (under 50 and over 50 years old) to limit the potential for confounding variables being assigned unequally to groups.35 Participants were then randomly allocated to intervention or control groups using computer-generated blocks of 6 or 8 to limit the possibility of chance imbalances with small samples. After all participants had been enrolled, a research assistant completed the randomization procedures and provided the research team with sealed sequentially numbered envelopes. The research team then advised participants of allocation to the intervention or control group.
Statistical Methods
Data were analyzed using intention to treat. Missing data were imputed using the last observation carried forward method, where missing values were replaced by the last-known value before the participant was lost to followup.36
At baseline, χ2 tests were used to compare groups for gender, family history, medications, and alternative therapies. Independent t-tests were used to compare age, duration of RLS, and HAP scores. Intervention and control groups were compared for primary and secondary outcomes at baseline using independent t-tests and post intervention (week 6) using ANCOVA37 with baseline scores as covariates in the model (α = 0.05). The magnitude of between-group differences was interpreted using the mean difference and its associated 95% CI.
Two-way ANOVAs (group × time), with repeated measures on time, were conducted to examine the effect of group, time, and group by time interactions on outcomes. Dunnett's correction for multiple comparisons was applied to maintain the family-wise error at an α of 0.05 when baseline values were compared with multiple follow-up assessments. Multiple comparisons were made to determine whether baseline scores differed significantly from measurements taken at each of the subsequent 6 weeks using multiplicity adjusted P values.
Sample size analysis was conducted to determine the likely outcomes had the sample been larger.
Results
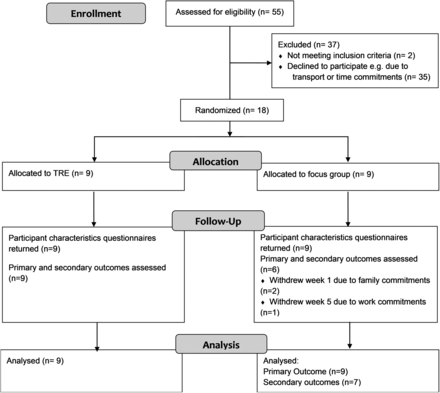
Figure 1 presents an overview of participant flow. Fifty-five participants expressed interest and were assessed for eligibility. Two participants were ineligible as they felt that they would not be physically able to complete the exercises. Eighteen participants were enrolled. As participants completed the online survey 1 to 2 weeks before commencement of the study, which included the IRLS but did not include secondary outcomes, only IRLS scores could be analyzed using imputation of missing data for the 2 participants who withdrew before week 1. All participants received intended treatment and were analyzed for the primary and secondary outcomes. The trial ran from March to May 2015.
Consort flowchart. TRE, trauma release exercises.
The intervention and control groups had attendance rates of 85% and 79%, respectively across the 6-week trial period. During the study, 62.5% of all participants experienced RLS symptoms in the legs only, while 37.5% of participants experienced symptoms in both the arms and the legs at some time during the study. On average, 65% of intervention group participants adhered to the recommended home exercise program. No participants withdrew due to harms. One participant in the intervention group experienced some emotional discomfort during the exercise program and was initially unable to control the tremoring. The participant was provided with individualized technique modification and debriefing, and was able to continue. No adverse events occurred in the control group.
Participant Characteristics
Participant characteristics are presented in Table 2. There were no significant between-group differences at baseline for any characteristic except for more women in the intervention group. Alternative therapies for RLS currently or previously used by participants included supplements such as magnesium, vitamins and fish oil, as well as yoga, chiropractic, massage, a homeopathic spray, and a low-FODMAP (low-fermentable oligosaccharides, disaccharides, and monosaccharides, and polyols) diet.38
Participant Characteristics at Baseline
Baseline Primary and Secondary Outcomes
Baseline data for primary and secondary outcomes are presented in Table 3. There were no significant between group differences at baseline on any outcome with the exception of global RLS (0 to 10) scores, with a significantly greater severity reported by control group participants.
Baseline IRLS, Global RLS, Global Stress, PIRS and MDI Scores for the Trauma Release Exercises (TRE) and Control Groups
Between-Group Analyses
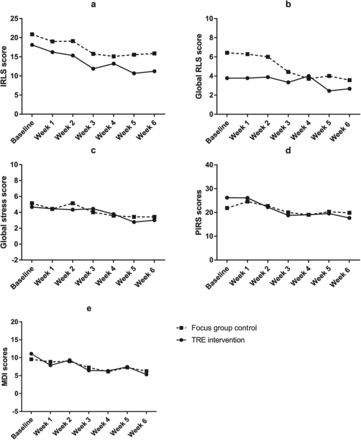
Figure 2 presents intervention and control group scores over time for each outcome.
Baseline to post-intervention plot of International Restless Legs Syndrome Rating Scale (IRLS), global restless legs syndrome (RLS), global stress, Pittsburgh Insomnia Rating Scale (PIRS), and Major Depression Inventory (MDI) scores for trauma release exercises (TRE) and control groups. A, IRLS, scores over time. B, global RLS (Visual Analogue Scale [VAS], 0 to 10) over time. C, global stress (VAS, 0 to 10) scores over time. D, PIRS scores over time. E, MDI scores over time.
Data for primary and secondary outcomes at 6 weeks are presented in Table 4. No significant between group differences were identified on any outcome after the intervention. There were no significant group by time interaction effects for any outcome. There were significant effects attributable to time on all outcomes.
Post-Intervention IRLS, Global Restless Legs Syndrome (RLS), Global Stress, PIRS, and MDI Scores for the Trauma Release Exercises (TRE) and Control Groups
The primary outcome of IRLS scores for both groups changed during the 6 weeks of the study (mean [SD] change scores intervention, 6.9 [5.7]; control, 5 [4.6]). Sample size estimates using these statistics indicated that around 234 participants would be required for these small between-group differences to achieve significance if a future study replicated this work.
Discussion
To our knowledge, this is the first trial exploring the effects of TRE for RLS. There were minimal associated harms with the intervention. There were no significant differences between intervention or control groups at week 6 on any outcome, and no significant group by time interaction effects on any outcome. This was unlikely to be due to the effect of small sample size on uncertainty in estimates, because both final outcomes, and the way that scores changed across time, were similar for both groups. These findings held for both primary and secondary outcomes. Although a criticism of this study might be the small sample size, it represents a responsible preliminary analysis of the likely benefits of conducting a larger study, particularly in the light of the speculative nature of claims regarding effects of TRE.
Despite small numbers, the study did reveal significant effects attributable to time for all outcomes. The uniform behavior of the intervention and control groups across time was particularly interesting. If the effects across time were due to the intervention (exercise based), then the control condition (nonexercise based) seems to have had very similar effects on RLS severity, sleep quality, and depression. According to Ondo et al,39 a 6-point change in IRLS score is clinically meaningful. In our study, 6 participants in the intervention group and 3 participants in the control group reported a change that could therefore be considered to be clinically meaningful. Important changes in symptoms were therefore not uniquely found in the intervention group.
Explanations for the similar changes across time in both groups include the possibility that participants changed the way they reported symptoms because they repeatedly completed the same assessment forms and gained familiarity with reporting procedures. The impact of assessment may have also changed participant perception of symptoms.40 Regression toward the mean41 might also explain observed effects. People may have considered participation in research into RLS because their symptoms were unusually severe. A decrease in severity would be expected following the point in time when severity was particularly high, providing the appearance of improvement attributable to study conditions. Future trials investigating therapy for RLS might include a prolonged baseline phase with monitoring to identify when scores stabilize. This would improve confidence in the likely sources of changes in scores across time.
Alternatively, the effects across time for both groups may have been attributable to the effect of the group environment. It is possible that informal or formal sharing of experiences and strategies for managing RLS over the 8 face-to-face hours of the study reduced perception of the problem, or increased confidence in managing the condition, resulting in participants in both groups reporting ongoing improvement during the study. Benefits of group support have been reported for others with chronic illness,42 particularly less well understood conditions such as chronic fatigue syndrome.43 There may be considerable benefit to improving our understanding about whether group interaction plays a role in therapeutic effects for people with RLS. Not only are the specific effects of interest in supporting people with this condition, but the impact of group interaction needs to be partitioned in outcomes analysis in future trials of therapies. None of the eleven randomized controlled trials investigating nonpharmacological therapies for RLS12⇓⇓⇓⇓⇓⇓⇓⇓⇓–22 have provided insight into the potential effects of group therapy on symptoms, so this seems to be an important target for future attention.
Bias was minimized in this study by addressing key criteria argued as essential in high-quality randomized controlled trials.44 Due to the nature of the intervention, participant and therapist blinding was not possible, and this meant that the self-reported outcome assessment was not blind.44 Estimations of RLS duration cannot be considered accurate given the poor recall of a number of participants, and therefore groups may have been different at baseline for duration of complaint. Generalizing these results to a different age group, RLS severity, or environment should be undertaken with caution.
Participants were stratified for RLS severity and age, however, not for gender, resulting in significant differences between groups for percentage of male and female participants. It is unlikely these differences affected RLS scores, as no evidence indicates gender-specific differences in clinical presentation of RLS.45 Based on outcome measurements, there appeared to be a lot of variability in the presentation of RLS, and this should be considered in addition to effect sizes when considering sample size estimates. A SD of 5.85 for IRLS scores was used in the power estimates, and the baseline SD in our combined sample was 5.95, suggesting that this was a reasonable parameter for sample size estimation. The last data point carried forward method for missing data may have introduced bias in analyzing data; however, conclusions did not differ when data were analyzed with and without imputed values.
Conclusions
Findings of this exploratory study provided preliminary data indicating that TRE and attending a series of discussion groups with other people with RLS were associated with similar outcomes. Both groups reported a reduction in RLS symptom severity and depression, and an improvement in sleep quality. The systematic reduction in RLS severity across the 6 weeks of the study highlights the need to collect baseline data over a prolonged period of time before intervening to counter the possible effects of regression to the mean and repeated assessment.
Acknowledgments
The authors are very grateful to those people who participated in this study. We particularly acknowledge Richmond Heath, who instigated the work, designed and delivered the trauma release exercise sessions, and was an insightful and supportive collaborator.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
To see this article online, please go to: http://jabfm.org/content/31/5/783.full.
- Received for publication February 23, 2018.
- Revision received May 11, 2018.
- Accepted for publication May 14, 2018.