Abstract
Introduction: Often misperceived as solely a dental disease, periodontitis is a chronic condition characterized by inflammation of the support structures of the tooth and associated with chronic systemic inflammation and endothelial dysfunction. Despite affecting almost 40% of US adults 30 years of age or older, periodontitis is rarely considered when quantifying the multimorbidity (the presence of 2 or more chronic conditions in an individual) burden for our patients. Multimorbidity represents a major challenge for primary care and is associated with increasing health care expenditure and increased hospitalizations. We hypothesized that periodontitis was associated with multimorbidity.
Methods: To interrogate our hypothesis, we performed a secondary data analysis of a population-based cross-sectional survey, the NHANES 2011 to 2014 dataset. The study population included US adults aged 30 years or older who underwent a periodontal examination. Prevalence of periodontitis in individuals with and without multimorbidity was calculated using likelihood estimates and adjusting for confounding variables with logistic regression models.
Results: Individuals with multimorbidity were more likely than the general population and individuals without multimorbidity to have periodontitis. However, in adjusted analyses, there was no independent association between periodontitis and multimorbidity. Given the absence of an association, we included periodontitis as a qualifying condition for the diagnosis of multimorbidity. As a result, the prevalence of multimorbidity in US adults 30 years and older increased from 54.1% to 65.8%.
Discussion: Periodontitis is a highly prevalent, preventable chronic inflammatory condition. It shares many common risk factors with multimorbidity but was not independently associated with multimorbidity in our study. Further research is required to understand these observations and whether treating periodontitis in patients with multimorbidity may improve health care outcomes.
Introduction
Primary care physicians are spending an increasing amount of time in chronic disease prevention and management, with approximately half of all Americans having at least 1 chronic illness.1 Periodontitis is one of the most common chronic systemic inflammatory diseases globally.2,3 While often viewed as exclusively a dental disease, periodontitis causes systemic inflammatory responses and endothelial dysfunction.4
Over 40% of US adults over 30 have periodontitis,5 a chronic condition characterized by inflammation of the supporting structures of the tooth. Presentations of periodontitis span a spectrum including asymptomatic; minor bleeding gums with toothbrushing; dental pain, tooth loss and deterioration in masticatory function resulting in poor nutrition. Risk factors for the development of periodontitis are similar to other chronic noncommunicable diseases and include age, male gender, smoking, obesity,6 and lower socioeconomic status.7
With an increase in longevity, and the prevalence of chronic conditions, medical health professionals have seen a rise in what the World Health Organization defines as multimorbidity (MM): the presence of 2 or more chronic conditions in the same individual.8 MM is associated with an array of poor health outcomes including hospitalizations and high health expenditure.9 Interestingly, studies rarely include periodontitis as a qualifying condition in studies quantifying multimorbidity prevalence.10
Caring for patients with multimorbidity in primary care is challenging for multiple reasons including, but not limited to balancing the uncertainties of applying disease specific guidelines for multiple coexistent conditions; risks of polypharmacy and facilitating shared decision making on evidence-based interventions.11,12
With Chronic Illness, Comes Increasing Health Care Costs and Related Burdens9
Periodontitis is associated with an array of chronic medical conditions, including both type 1 and type 2 diabetes,13 inflammatory bowel disease,14 and cardiovascular disease4—to name just a few. Periodontitis treatment in individuals with type 2 diabetes has been shown to lead to improvements in glycemic control suggesting the possibility that interventions that impact periodontitis may lead to systemic improvements in health.15,16 In addition, periodontitis is associated with significant economic burden17 and is potentially a modifiable predictor of excess medical expenditure.18 Periodontitis treatment in individuals with diabetes and cardiovascular disease is associated with lower hospitalizations and health care costs.19
Given the high prevalence of periodontitis and associations with several chronic conditions, our hypothesis is that periodontitis is highly prevalent among individuals with multimorbidity. By understanding the prevalence of periodontitis among individuals with multimorbidity, we hope to address a major gap in the field and begin to better identify those for whom aggressive screening for and management of periodontitis may be beneficial.
Methods
The authors conducted a secondary data analysis of the National Health and Nutrition Examination Survey (NHANES) data from 2011 to 2014. The NHANES is a stratified multistage probability sample of the noninstitutionalized US population with each 2-year survey cycle examining approximately 10,000 persons and collecting health-related data. Full descriptions of sampling and design for all NHANES datasets are publicly available. In this study, demographic, questionnaire, medical examination, and periodontal examination data from 2 NHANES cohorts (2011 to 2012), (2013 to 2014) were extracted and merged to comprise the study population. Subsequent NHANES did not include a full mouth periodontal examination, so the 2011 to 2014 data represents the most up to date dataset.
Inclusion Criteria
Only adults aged 30 years or older were eligible for a periodontal examination, provided they did not require antibiotic prophylaxis before periodontal examination and had 1 or more natural teeth. We therefore limited our analyses to adults 30 years or older who underwent a periodontal examination.
Demographics of Participant Cohort
Demographic variables of interest included: Age, gender (Male or Female), smoking status (not at all, some days, or every day), alcohol consumption (averages ≥2 drinks a day or ≤2 drinks a day), health insurance status (has coverage or does not have coverage), household income (monthly income: low ranged from $0 to $1649, moderate ranged from $1650 to $4599, and high was ≥$4600), education level (less than 9th grade, 9th to 11th grade, high school diploma or equivalent, some college/associate’s degree, or bachelor’s degree or higher), racial/ethnic affiliation (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, or other race), marital status (married, widowed, divorced, separated, never married, or living with partner), prescription numbers (0 to 1, 2 to 4, 5 to 10, or ≥11), dental and health service utilization patterns, and medical conditions were included from the NHANES survey data. Dental and health service utilization were measured by time of last dentist visit (0 to 6 months, 6 months to 1 year, 1 to 2 years, 2 to 3 years, 3 to 5 years, more than 5 years, or never) and if the person needed dental care in the last year but could not get that care (yes or no).
Definition of Multimorbidity
MM was defined as the presence of 2 or more chronic conditions in the same individual. A validated MM tool for self-reported health conditions in primary care was used to determine qualifying chronic conditions.20 The NHANES surveys collected data on 17 of the 20 qualifying chronic conditions (Appendix 1). Of note the NHANES data did not differentiate between type 1 or type 2 diabetes so were all were grouped under diabetes.
Periodontal Examinations
All periodontal examinations were performed by qualified general dentists, trained in the study protocol in NHANES 2011 to 2014. Periodontitis status was assessed by means of a full-mouth periodontal examination (FMPE) at 6 sites per tooth on all non-third molar teeth. FMPE is the gold standard for diagnosis of periodontitis.21 A minimum of 25 examinations per dentist were repeated annually for quality assurance and the subsequent quality assurance assessment demonstrated a high data quality level and ‘substantial’ examiner reliability for periodontitis determination.22
Periodontitis Case Definition
Standard periodontitis case definitions as defined by the Centers for Disease Control and Prevention (CDC) and the American Academy of Periodontology were used for this study. A health technician entered all examiner observations directly into the data collection system and the published coding algorithm was used to categorize based on periodontitis presence or absence and subcategories of severity (Appendix 2).
Statistical Analyses
Missing data were a concern in the analysis since certain variables of interest had high percentages of missingness. The covariates with the highest percentage missing were smoking status (57% missing), alcohol consumption (37%), diabetes (16%), and income (12%). Data were also missing in education level (0.1%), marital status (0.1%), time since last dentist visit (0.1%), needed dental care but could not get it (1%), sleep disorder (0.2%), and health insurance (0.03%). Multiple imputation by chained equations23 was used to impute values for these missing covariates. The fully observed covariates age (continuous), gender, race/ethnicity, prescription count, and MM were also included in the imputation models as well as an interaction between (categorical) age and MM. Age was categorized as 30 to 39, 40 to 50, 50 to 64, or 65 and older. Using PROC MI in SAS 9.4, 25 imputed datasets were generated.
A weighted logistic regression model was used to investigate the association between the outcome of any periodontitis with multiple morbidity (MM) adjusting for the following covariates: categorical age (reference category 30 to 39), gender (reference: female), race/ethnicity (reference: non-Hispanic White), education (reference: college grad or above), income (reference: high), insurance (reference: yes), smoking (reference: not at all), and alcohol consumption (reference: less than 2 drinks/day). A model with an interaction between MM and age was also fit to the data. The aforementioned weighted logistic regression models were fit to each of the 25 imputed datasets separately using PROC SURVEYLOGISTIC in SAS. The survey weights are provided in the NHANES data.24 Estimates from these 25 model fits were combined using PROC MIANALYZE to give estimates for the associations of periodontitis and the covariates which account for the uncertainty in the imputed datasets. The combined estimates, P values, odds ratios (ORs), and 95% confidence intervals (CIs) for the OR were all reported.
The CDC Ethics Board approved the oral health examination protocol and all patients provided written informed consent. As a secondary data analysis of publicly available datasets, the study was deemed not regulated by the institutional review board of the University of Michigan.
Results
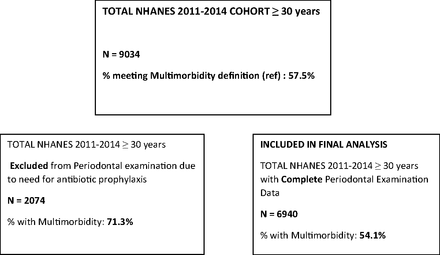
The total combined NHANES 2011 to 2014 sample included 9034 individuals 30 years or older. Using a validated tool for the diagnosis of multimorbidity,20 the weighted prevalence of multimorbidity among US adults greater than 30 years of age who participated in the NHANES 2011 to 2014 was 57.5%. Individuals 30 years or older with available, complete periodontal examination data were included in the final sample: 6940 US adults (3323 from NHANES 2011 to 2012 and 3617 from NHANES 2013 to 2014). The weighted prevalence of multimorbidity in this cohort of participants was 54.1% (Figure 1).
Schematic representation of differences in multimorbidity prevalence of individuals excluded from periodontal examination.
Participants with multimorbidity who underwent a complete periodontal examination had important demographic differences to those without multimorbidity (Table 1). Participants with MM were older, with a mean age of 55.9 and were more likely to be female. Participants with multimorbidity were also more likely to be non-Hispanic white, have low household income and less likely to have a college degree or higher. In addition, participants with MM were less likely to be everyday smokers and more likely to have health insurance and to have seen a dentist within the past year (Table 1).
Selected Covariates by Multimorbidity (in Weighted Percentages)* in Observed Data
The prevalence of periodontitis in the NHANES 2011 to 2014 was 39.9%, with 7.5% of cases classified as severe periodontitis. Among study participants with periodontitis, there were significant demographic differences when compared with those without periodontitis (Table 2). Participants with periodontitis were of older age, more likely to be male and of non-Hispanic black or Mexican American ethnicity. These participants were also less likely to have a college degree or higher and more likely to have low monthly household income and be current everyday smokers. These periodontitis-laden participants were additionally more likely to consume 2 or more alcohol beverages daily and were less likely to have health insurance and to have seen a dentist within the past year (Table 2).
Selected Covariates by Periodontitis (in Weighted Percentages)* in Observed Data
Periodontitis was associated with a variety of chronic conditions in this study including obesity, cardiovascular disease, heart failure, diabetes, depression, arthritis, kidney disease and stroke. However, when we controlled for confounders, only associations between periodontitis and cardiovascular diseases, heart failure and diabetes remained statistically significant (Table 3).
Weighted Percentages of Chronic Conditions by Periodontitis Status
Periodontitis Was Not Independently Associated With Multimorbidity
To investigate the hypothesis that multimorbidity is independently associated with periodontitis we performed logistic regression analyses. In unadjusted analyses, participants with multimorbidity had a higher prevalence of periodontitis than those without multimorbidity (42.9% vs 36.4%, P < .0001). We concluded that in unadjusted analysis, multimorbidity was associated with an increased odds of periodontitis Given the association noted above between multimorbidity and periodontitis, these associations were examined in analyses adjusting for potential confounders. A weighted logistic regression model was adjusted for potential confounders including age, gender, race/ethnicity, education, income, insurance, smoking, and alcohol consumption. An interaction between MM and age-group was found to not be statistically significant (results not shown). We report results for the weighted logistic model with main effects only. In this adjusted analysis, multimorbidity was not independently associated with periodontitis (adjusted OR 0.98; 95% CI 0.91, 1.06; Table 4). In addition, individuals with multimorbidity did not have higher prevalence of severe periodontitis than those without multimorbidity (unadjusted, 7.1% vs 8.0%; P = 0.22).
Logistic Regression Results From Weighted Logistic Models on 25 Imputed Datasets
Multimorbidity Prevalence Increases When Periodontitis is Counted as a Chronic Condition
When we included periodontitis as a qualifying chronic condition for the definition of multimorbidity in our study, the weighted prevalence of multimorbidity in NHANES 2011–2014 rose from 54.1% to 65.8% in the study cohort who had complete periodontal examination data.
Discussion
This study highlights the high prevalence of both MM (57.5% in US adults ≥30; 54.1% among those who had periodontal examinations) and periodontitis (39.9%) in US adults over the age of 30. The presence of periodontitis or multimorbidity was associated with multiple modifiable risk factors such as smoking, alcohol, lower socioeconomic status (Tables 1 and 2).
While the prevalence of periodontitis is higher among individuals with multimorbidity compared with individuals without multimorbidity (42.9% vs 36.4%, P < .0001), there was no independent association found when adjusting for cofounding variables. In addition, participants with multimorbidity were not at increased risk of more severe periodontitis than those without multimorbidity. This is significant as it clarifies that periodontitis is an important separate condition, not merely a comorbid or complicating condition for the index MM conditions.
Despite being the 11th most common chronic systemic inflammatory disease globally, periodontitis is rarely included in studies quantifying multimorbidity prevalence.25 In this study when periodontitis was included as a qualifying chronic condition under the definition of multimorbidity, the weighted prevalence of multimorbidity in NHANES 2011 to 2014 increased from 54.1% to 65.8%, in the study cohort who had complete periodontal examination data. The population prevalence of adults ≥30 with at least 1 chronic health condition rises from 79.9% to 86.7% by including periodontitis. Including periodontitis routinely in quantification and discussion of multimorbidity burden is important for several reasons. Improved awareness of the importance of periodontal health can benefit patients, help promote enhanced care coordination between dental and medical care, help advocate for improved affordability and access to dental care for at risk patients and potential impact overall systemic well-being and health care costs.
We believe it is important for family physicians to understand that periodontitis is a separate chronic systemic inflammatory condition, however, it co-occurs frequently in our patients with multimorbidity. For patients with type 2 diabetes and periodontitis, treating periodontitis has been shown to improve glycemic control. Recommending attention to good oral hygiene and routinely seeing a dentist as part of the annual health maintenance examination is a simple strategy that can help optimize oral health in individuals both with and without multimorbidity.26,27 The impact of treatment of periodontitis in patients with multimorbidity both in terms of their oral and their systemic health deserves further study.
In this study, participants with multimorbidity were more likely to have health insurance and to have seen a dentist within the past year. Access to dental care provides opportunities for prevention, early diagnosis and treatment of periodontitis which may lead to improved periodontal health in patients with multimorbidity. Periodontitis can be effectively treated by general dentists as well as periodontal specialists using common in office dental techniques including scaling and root planing, so it is important that all patients have access to timely and affordable dental care.
Conversely, participants who had periodontitis in this study were less likely to have health insurance and significantly less likely to have seen a dentist within the past year. A striking 25.1% of people with periodontitis in our study did not have health insurance. Participants without health insurance may have undiagnosed chronic conditions and as this study relied on patient self-reported chronic conditions, the true multimorbidity burden among patients found to have periodontitis on the NHANES oral examination may be higher than reported.
Periodontitis and Chronic Disease Associations
Periodontitis has been associated with a wide variety of chronic conditions including both type 1 and type 2 diabetes, rheumatoid arthritis, inflammatory bowel disease,14 cardiovascular diseases, kidney disease,28 and depression as well as adverse pregnancy outcomes.3,29–31 This study highlights the known association between periodontitis and several individual chronic conditions (Table 3). Diabetes, heart failure and cardiovascular disease were all independently associated with periodontitis after adjusting for confounders. One unexpected observation from this research was that there was no association between osteoporosis and periodontitis - despite biological plausibility and literature broadly supportive of this association. This study did not account for osteoporosis treatment which may be a confounding factor.
When we consider patients with type 2 diabetes and periodontitis, there are proven benefits from periodontal treatment in these patients in terms of both periodontal disease improvement and improvement in glycemic targets.32 The exact mechanism remains to be elucidated but improved periodontal disease and improved glycemic control both lead to improved patient-centered outcomes. Jeffcoat and colleagues’19 observational findings of reduced hospitalizations and health care costs associated with treatment of periodontitis in individuals with type 2 diabetes and cardiovascular diseases are potentially very exciting but remain to be studied in a randomized controlled trial setting.
This study was a secondary data analysis of a large cross-sectional study using a publicly available data set. We utilized Fortin and colleagues20 validated multimorbidity tool for defining MM in our study given concerns in the literature about the wide variety of definitions and qualifying conditions included in multimorbidity research.10 The diagnosis of periodontitis in this study was based on the gold standard full mouth periodontal examination by qualified general dentists in a high-quality controlled setting as part of the NHANES 2011 to 2014.
There are limitations inherent in all secondary data analyses. In this study, the MM conditions were by self-report, with not all included survey items formally validated. The NHANES medical categories were not exact matches with Fortin and colleagues condition descriptors, with no data available for 3 of Fortin’s 20 categories (Appendix 1).20 This may have led to underestimation of true multimorbidity prevalence, however our population estimates are in line with US published multimorbidity prevalence data.33 All adults over 30 who had a health condition which necessitated antibiotic prophylaxis before an oral health examination were excluded from undergoing a periodontal examination by the NHANES investigators. There were 2074 US adults over 30 during NHANES 2011 to 2014 who did not undergo an oral health examination. There was a significantly higher prevalence of multimorbidity in those who did not undergo an oral health examination (71.3% vs 54.1%, P < .0001; Figure 1). This may have resulted in the exclusion of people with higher multimorbidity burden from our study. It may also suggest that the population prevalence of periodontitis may be even higher than estimated in NHANES.
Conclusions
This study demonstrates a high prevalence of periodontitis among patients with multimorbidity, higher than the general population and in those without multimorbidity. Periodontitis and multimorbidity were not independently associated in our study bolstering our argument that a diagnosis of periodontitis needs to be considered when quantifying multimorbidity. More research is needed to determine if finding and treating periodontitis in our patients with multimorbidity may lead to systemic health benefits beyond improved periodontal outcomes alone.
Acknowledgments
Special thanks to Dr. Julianne Cronin, BDS, Health Service Executive South, Ireland for periodontitis expertise and to Dr. Mas Jimbo, Dr. Suzanne Zick, Dr. Diane Harper and Dr. Sherri Sheinfeld Gorin of the Faculty Development Institute for their mentorship during the research process.
Appendix.
Appendix 1. Qualifying Conditions From Fortin et al. and Corresponding National Health and Nutrition Examination Survey (NHANES) Categories
Appendix 2.
The published algorithm has a typographical error in the code. The CDC definition of moderate periodontitis is ≥2 interproximal sites with ≥4 mm LOA or ≥2 interproximal sites with ≥5 mm PD.
In the code there are the variables: lobsum = number of sites with LOA ≥4 mm; pdsum = # sites with PD ≥5 mm. There is also a variable loasum = number sites with LOA ≥6 mm (which is used to define severe periodontitis).
In their code, when they determine if there is moderate periodontitis, they check if loasum ≥2 or pdsum ≥2 but it should be if lobsum ≥2 or pdsum ≥2.
Notes
This article was externally peer reviewed.
Competing interests: None.
Funding: None.
To see this article online, please go to: http://jabfm.org/content/36/2/313.full.
- Received for publication June 9, 2022.
- Revision received November 21, 2022.
- Accepted for publication November 28, 2022.