Abstract
Background: Primary care providers (PCPs) frequently address dermatologic concerns and perform skin examinations during clinical encounters. For PCPs who evaluate concerning skin lesions, dermoscopy (a noninvasive skin visualization technique) has been shown to increase the sensitivity for skin cancer diagnosis compared with unassisted clinical examinations. Because no formal consensus existed on the fundamental knowledge and skills that PCPs should have with respect to dermoscopy for skin cancer detection, the objective of this study was to develop an expert consensus statement on proficiency standards for PCPs learning or using dermoscopy.
Methods: A 2-phase modified Delphi method was used to develop 2 proficiency standards. In the study’s first phase, a focus group of PCPs and dermatologists generated a list of dermoscopic diagnoses and associated features. In the second phase, a larger panel evaluated the proposed list and determined whether each diagnosis was reflective of a foundational or intermediate proficiency or neither.
Results: Of the 35 initial panelists, 5 PCPs were lost to follow-up or withdrew; 30 completed the fifth and last round. The final consensus-based list contained 39 dermoscopic diagnoses and associated features.
Conclusions: This consensus statement will inform the development of PCP-targeted dermoscopy training initiatives designed to support early cancer detection.
- Continuing Medical Education
- Delphi Method
- Dermoscopy
- Expert Opinion
- Focus Groups
- General Practitioners
- Melanoma
- Primary Care Physicians
- Primary Health Care
- Skin Cancer
Background
Skin cancer is the most common cancer in the United States, and the 3 major types are basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and melanoma. While most BCCs and SCCs are treatable and curable, melanoma is fatal when detected at advanced stages.1,2 Delays in diagnosis and treatment can be caused by lack of timely recognition exacerbated by poor access to dermatology specialists for evaluation of skin lesions. In the United States, these access disparities occur along the lines of patient socioeconomic status, race/ethnicity, and rural residence.3,4 In regions with barriers to dermatology access, trained primary care providers (PCPs) including advanced practice practitioners, such as physician assistants and nurse practitioners, play an important role in the detection, diagnosis, and management of skin cancer.5
For the early detection of skin cancer, clinical skin examinations are 1 of the safest and most cost-effective screening interventions available to patients.6 Skin examinations may be performed unassisted (with the naked eye) or with dermoscopy, a visualization technique involving use of a dermatoscope. A dermatoscope is a handheld instrument consisting of a magnifier and a polarized light source that enables detailed examination of surface and subsurface features not discernible by the naked eye.7 Dermoscopy use results in a higher diagnostic accuracy for melanoma detection compared with unassisted examinations.8 In a large meta-analysis of 104 published studies, dermoscopy was shown to significantly improve both the sensitivity and specificity for melanoma diagnosis when compared with visual inspection alone.9 This significantly reduces the number of melanomas overlooked and the number of benign lesions unnecessarily biopsied in the course of identifying melanoma, reducing patient morbidity and mortality.
On the frontline of health care delivery, PCPs frequently address dermatologic problems and perform skin examinations,10 and an estimated 12% to 25% of primary care encounters address a patient’s dermatologic problem.11,12 In a population-based study, 65.1% of patients presenting to their PCPs with skin-related issues did not seek further dermatologic care from a dermatologist or other health care provider that year.11 For patients at risk for skin cancer, each of these encounters in the primary care setting represents an opportunity to detect skin cancer at an early stage.
Among PCPs who treat skin conditions, appropriate training in the dermoscopic evaluation of skin lesions has been shown to improve their diagnostic sensitivity for skin cancer, including melanoma.13⇓⇓⇓–17 To gain proficiency in dermoscopy, clinicians must become familiar with the dermoscopic features (eg, colors, structures, patterns) of common dermatologic diagnoses.18 The recognition of these features supports a clinician’s decision of whether to biopsy, refer, or offer reassurance.
Before this study, no formal consensus existed on the fundamental competencies that PCPs should have with respect to dermoscopy for skin cancer detection.19,20 While a foundational dermoscopy proficiency standard has been developed for dermatology residents,21 the practice needs of PCPs differ from those of dermatologists, warranting a focused effort tailored to the primary care context. Therefore, the objective of this study was to develop an expert consensus statement on proficiency standards for PCPs learning or using dermoscopy.
To achieve this, the research team coordinated a modified Delphi exercise, an iterative method commonly used to obtain consensus opinion from a group of subject matter experts.22,23 For each proficiency standard, the expert panel determined which diagnoses and features are important for PCPs to identify, informing learner expectations for dermoscopy educators. In seeking agreement on specific competencies, this study will also establish content validity24 for PCP-targeted dermoscopy training programs and proficiency assessments.
Methods
Study Design
This study received approval from the MD Anderson Cancer Center Institutional Review Board (Protocol #2020-0667). The consensus process, as shown in Figure 1, used a 2-phase modified Delphi method for both the diagnoses and features stages. In the first phase, a smaller focus group generated a preliminary statement, and in the second phase, a larger panel refined the proposed statement through a controlled feedback process.21 This structured method guarantees that outcomes most closely represent the collective viewpoints of the group.22,23 To ensure anonymity of panelists, the research team administered electronic surveys using the web-based platform REDCap (Version 12.2.6, Vanderbilt University, Nashville, TN).
- Download figure
- Open in new tab
Consensus process using a 2-phase modified Delphi method.
This consensus process was organized as 2 successive stages: (1) a diagnoses survey series, and (2) a features survey series. To steer the consensus process, a focus group of 5 experts was assembled: 3 PCPs (PRC, AV & MDL) who routinely use dermoscopy in clinical practice and 2 pigmented lesion experts (EVS & KCN) who are highly engaged in PCP dermoscopy training initiatives. The focus group convened virtually before each survey series to propose, discuss, and approve survey items.
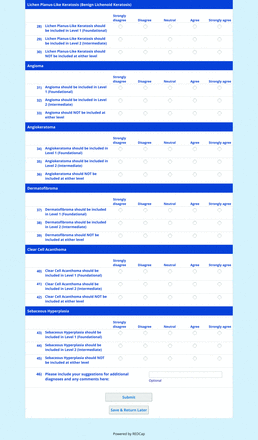
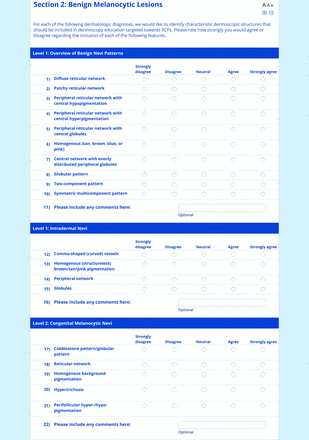
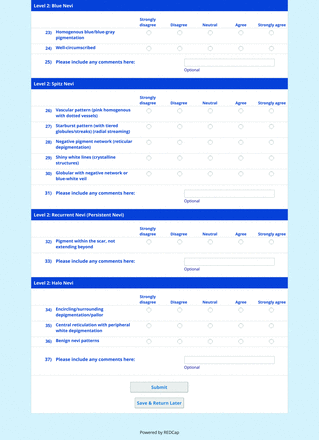
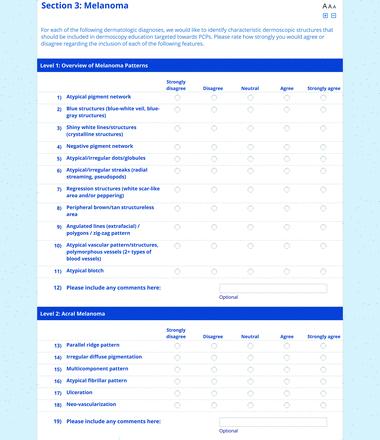
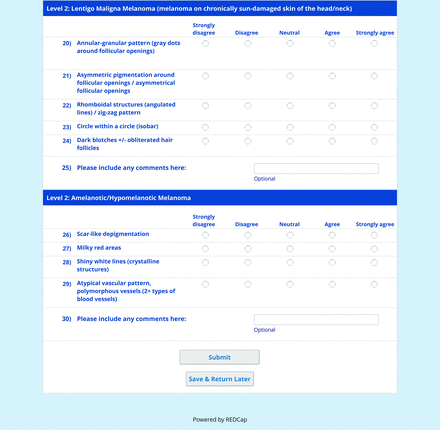
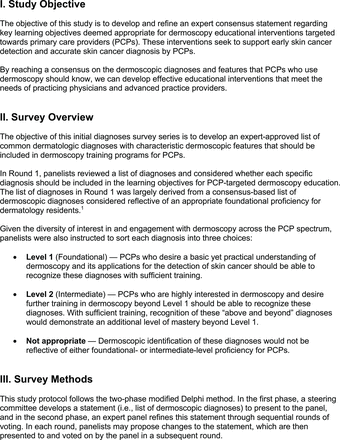
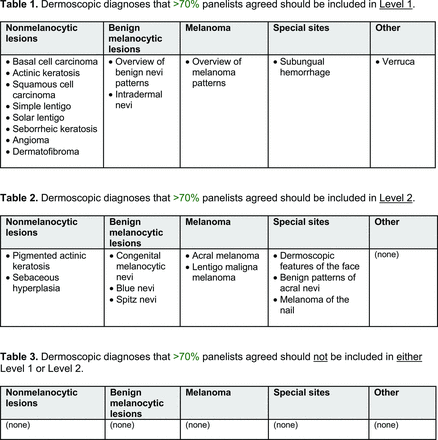
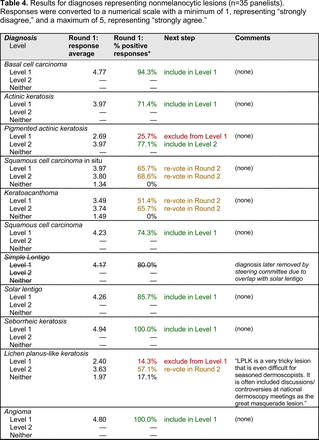
For the diagnoses survey series, the objective was to create an expert-approved list of common dermatologic diagnoses with characteristic dermoscopic features that should be included in dermoscopy training for PCPs. In the initial round, the panel reviewed a proposed list of diagnoses developed by the focus group. Most items on the list were drawn from a prior modified Delphi study that generated a foundational dermoscopy proficiency standard for dermatology residents.21 Contributors to this prior effort included members of the Melanoma Prevention Working Group–Pigmented Lesion Subcommittee (MPWG-PLS, affiliated with the Southwest Oncology Group and the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network) and other pigmented lesion experts.21
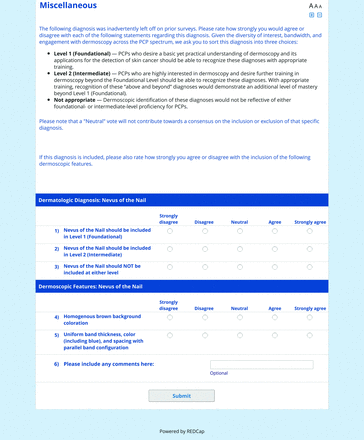
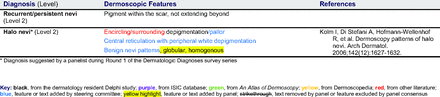
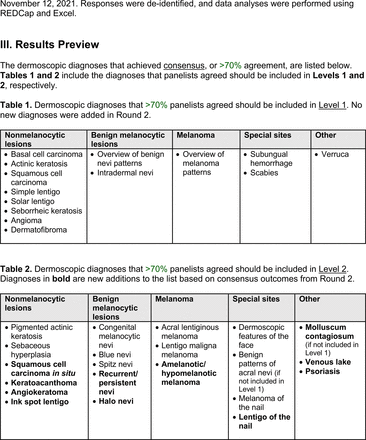
The proposed list was divided into 5 categories: nonmelanocytic lesions, benign melanocytic lesions, melanoma, special sites, and other diagnoses such as skin infections and infestations.21 This last category encompassed additional diagnoses (eg, verruca, molluscum contagiosum) that PCPs frequently encounter in clinical practice. Given the range of interest in and engagement with dermoscopy among PCPs, panelists were asked to assign each diagnosis to 1 of the following 3 options:
Level 1 (foundational): Clinicians who desire a basic yet practical understanding of dermoscopy and its application for skin cancer detection should be able to recognize these diagnoses with basic training.
Level 2 (intermediate): More experienced clinicians who are highly interested in learning dermoscopy beyond level 1 should be able to recognize these diagnoses. With adequate training, recognition of these “above and beyond” diagnoses would demonstrate an additional level of mastery beyond level 1. Diagnoses that do not reach consensus for inclusion in level 1 may be considered for inclusion in level 2.
Neither level 1 nor level 2: Recognition of these diagnoses using dermoscopy would not reflect either foundational or intermediate dermoscopy proficiency for PCPs. This may include diagnoses that are extremely rare in the population or that are especially challenging to diagnose, even by advanced dermoscopy users.
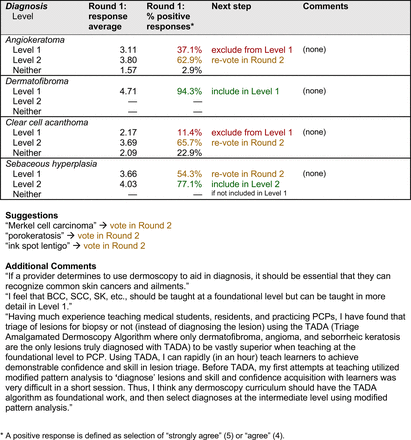
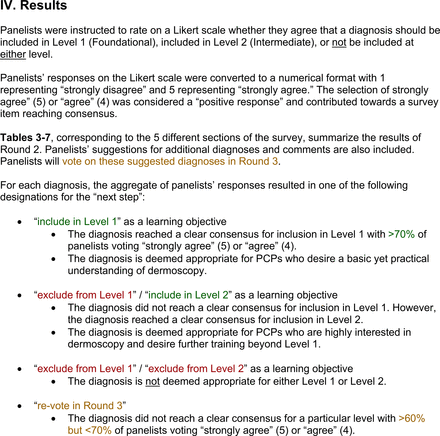
For each diagnosis, panelists rated how strongly they agreed or disagreed (via a 5-point Likert scale) with its inclusion in level 1, level 2, or neither. Panelists were also able to provide written feedback or suggest additional diagnoses to be presented in the next round. In subsequent rounds, panelists rerated diagnoses that nearly reached consensus for inclusion at a particular level (positive responses from >50% to 60% but <70% of participating panelists). Panelists also assigned additional diagnoses to level 1, level 2, or neither. Three formal rounds of surveys were performed between October and December 2021 until all diagnoses received a consensus-based assignment.
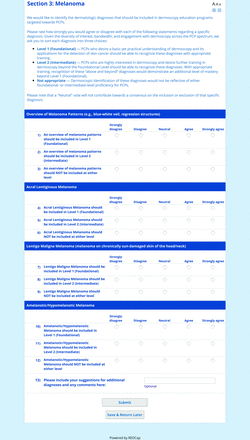
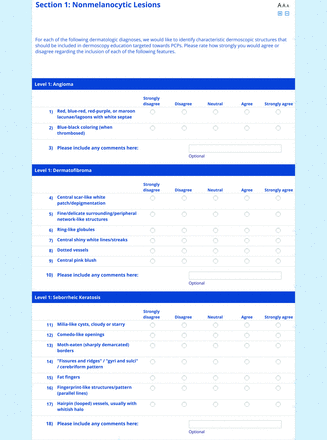
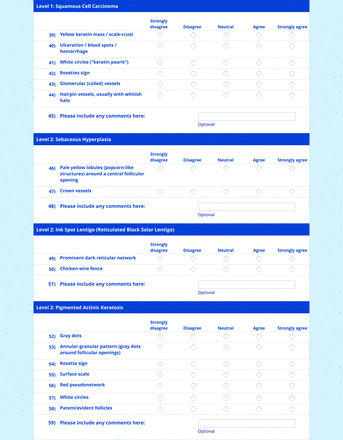
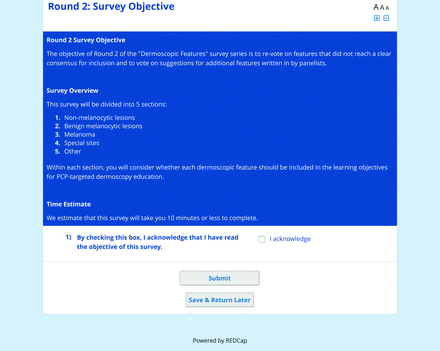
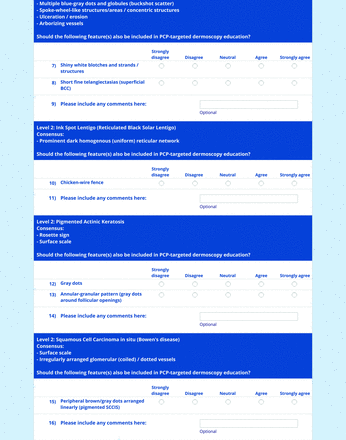
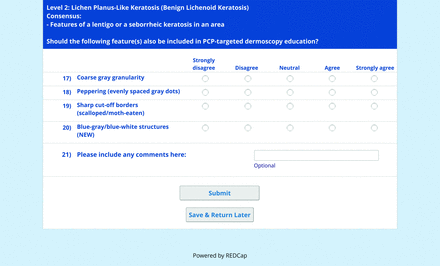
For the features survey series, the objective was to develop an expert-approved list of dermoscopic features for each included diagnosis. The aim was to capture features that are highly characteristic and important to recognize and that should be included in PCP dermoscopy education. Commonly seen structures may be included even if not specific to that diagnosis.
Based on a literature review, a proposed list of features was developed by the steering committee and presented to the panel. References for this list included the MPWG-PLS consensus on dermoscopy proficiency expectations for dermatology residents,21 the Dermoscopedia website,25 the 2016 International Dermoscopy Society consensus on dermoscopy terminology,26 the International Skin Imaging Collaboration dictionary of standardized terms, and other medical literature on PubMed, as documented in the online appendices.
For each feature, panelists rated on a 5-point Likert scale how strongly they would agree or disagree with its inclusion in dermoscopy training for primary care. Panelists were also able to propose wording modifications or suggest additional features. In the subsequent round, panelists rerated features that nearly reached consensus (positive responses from >60% but <70% of participating panelists) and rated additional features. Two formal rounds of features surveys were performed between December 2021 and February 2022.
On the conclusion of each round, all responses were deidentified, and data analyses were performed using REDCap and Microsoft Excel. Panelists received a summary of the preliminary results that reported the percentage of positive responses for each diagnosis. These results summaries were intended to inform panelists’ decisions in subsequent rounds.
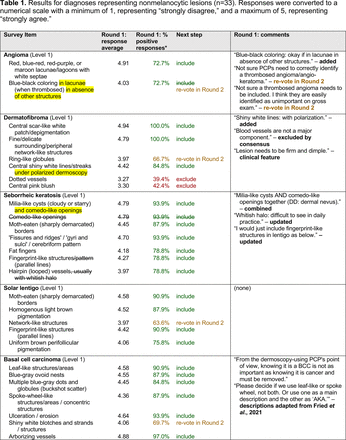
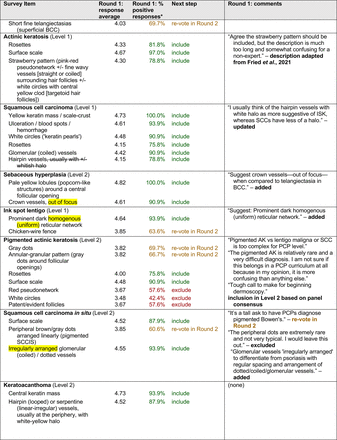
Each specific item that reached final consensus for inclusion received positive responses (defined as selection of “strongly agree” or “agree” on the Likert scale) from >70% of participating panelists. This threshold criterion was derived from the MPWG-PLS’s consensus process that used a similar 2-phase modified Delphi method.21 Features that received >50% but <70% positive responses were not formally included in the final consensus statement but were labeled as “optional to include” for PCP-targeted dermoscopy training.
Panel Recruitment
Through known professional networks, 40 subject matter experts were invited to join the panel: 25 PCPs (23 family medicine physicians and 2 internal medicine physicians) who routinely use dermoscopy in clinical practice and 15 dermatologists. Of the 15 invited dermatologists, most are directly involved in dermoscopy education and skin cancer detection training for PCPs, and 2 previously worked in primary care.
At the beginning of each survey, panelists reviewed and acknowledged a consent statement. No monetary compensation for panel participation was offered. For both survey series, copies of the consent statement, survey instruments, and results summaries can be found in the online appendices.
Results
Panelist Demographics
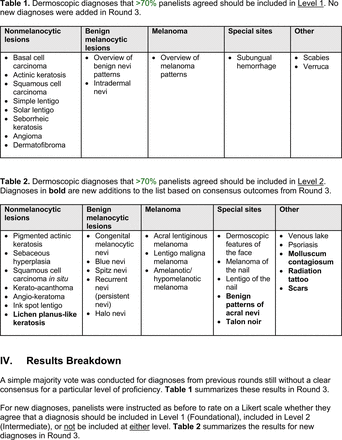
Of the 40 active physicians invited to join the panel, 35 (87.5%) participated in the initial round (Table 1). Of these 35, 21 (60.0%, 19 family medicine physicians and 2 internal medicine physicians) were PCPs (76.2% response rate), and the remaining 14 (40.0%) were dermatologists (93.3% response rate). Sixteen of the initial panelists (45.7%) reported specializing in pigmented lesions, dermoscopy, or melanoma as an attending physician. Of these 16, 3 were PCPs (2 family medicine physicians and 1 internal medicine physician), while the remainder were dermatologists. A majority (62.9%) reported being directly involved in dermoscopy training for primary care, offering training in the clinic and/or through lectures.
Demographic Characteristics of Larger Expert Panel (n = 35 Participants in First Round)
Over the course of the study, 5 PCPs were lost to follow-up or withdrew from the study. Of the 30 who completed the fifth and last round, 16 (53.3%, 14 family medicine physicians and 2 internal medicine physicians) were PCPs (76.2% retention rate), and 14 (46.7%) were dermatologists (100% retention rate).
Survey Results
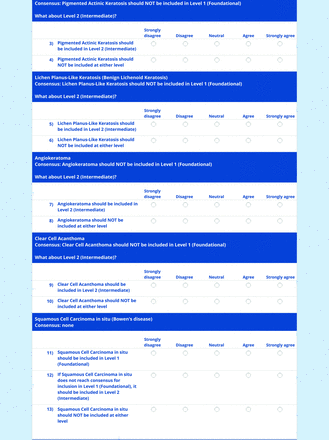
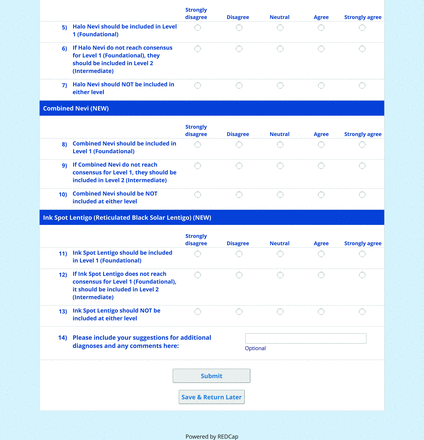
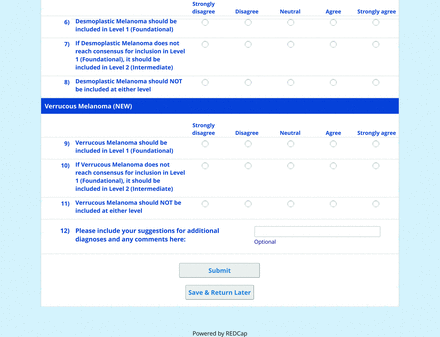
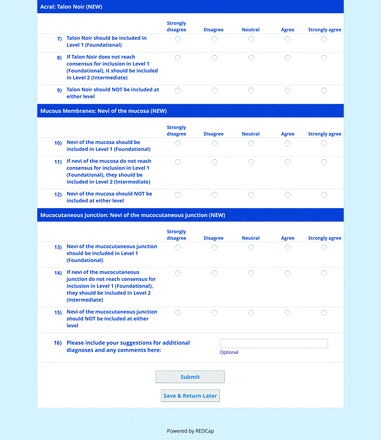
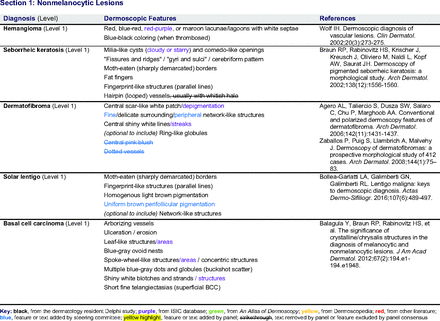
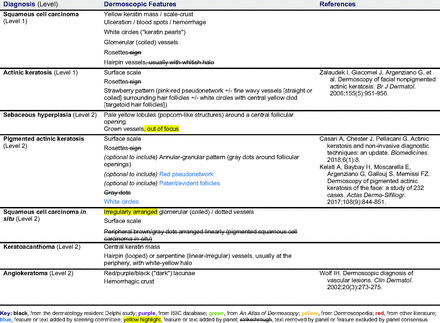
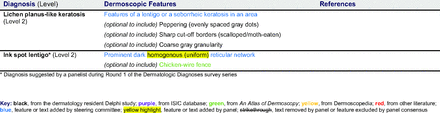
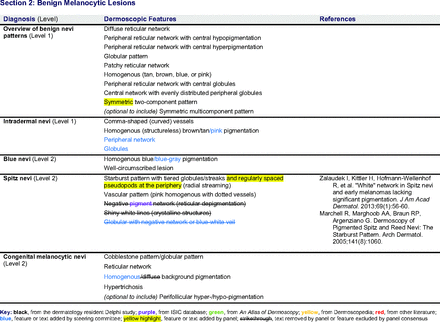
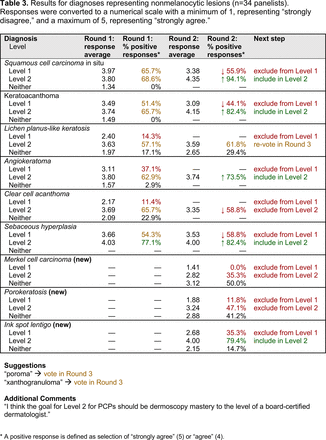
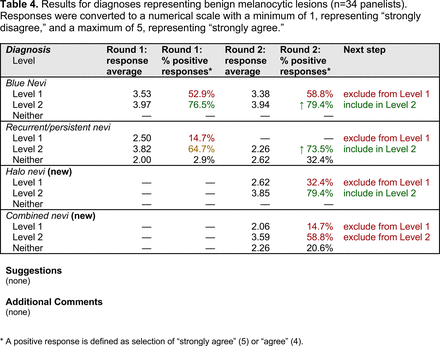
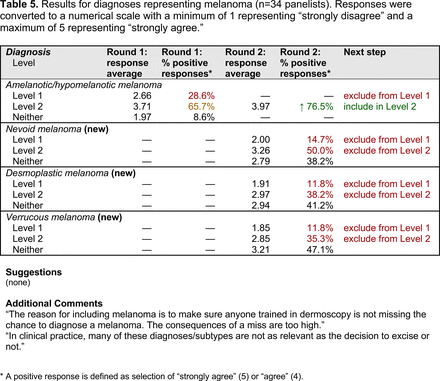
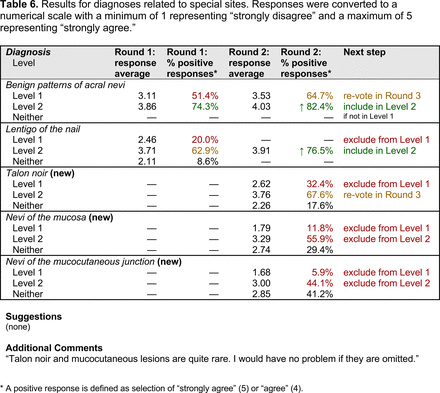
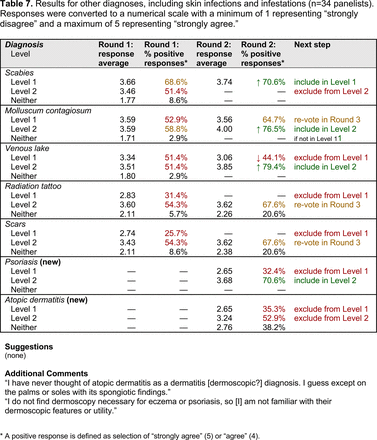
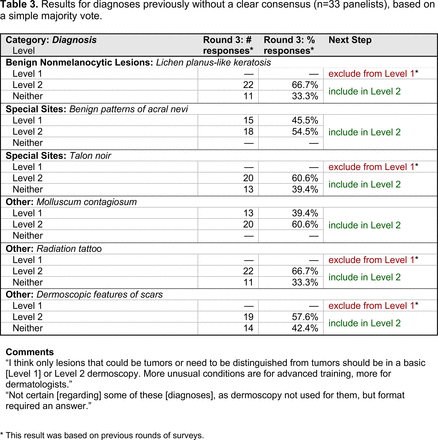
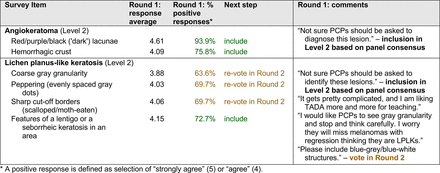
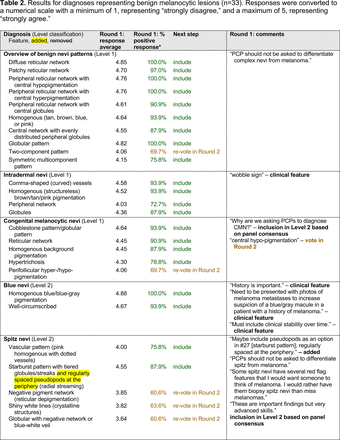
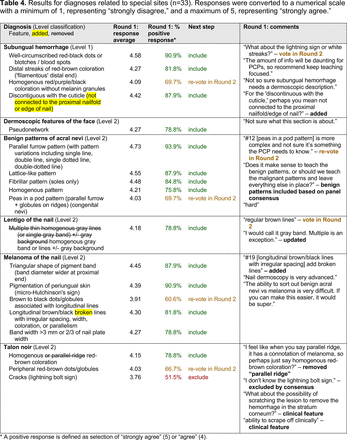
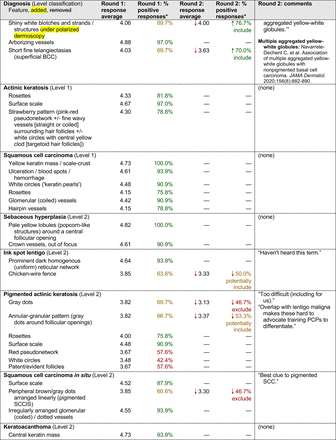
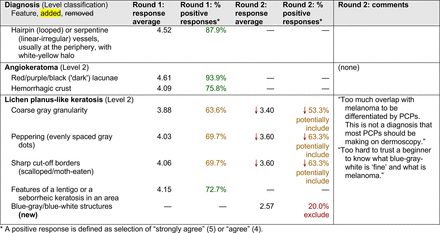
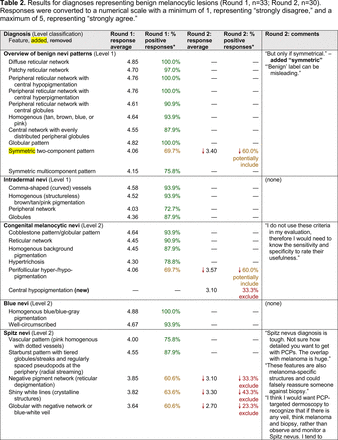
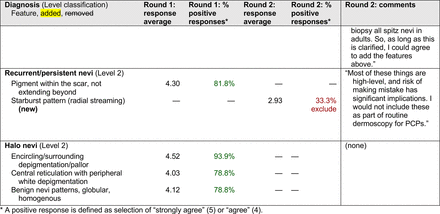
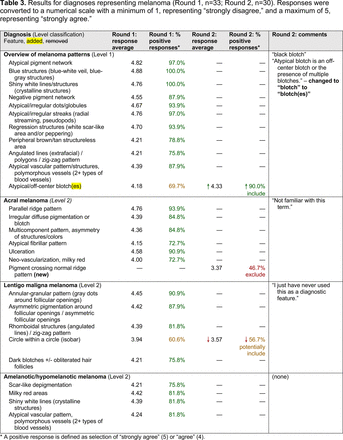
The consensus process involved 2 successive survey series: (1) diagnoses, and (2) features. In the diagnoses survey series, panelists voted on a total of 51 diagnoses (Table 2). Of this total, 15 represented additional diagnoses written in by panelists, and 39 received >70% positive responses and reached final consensus for inclusion (13 in level 1 and 26 in level 2).
Dermoscopic Diagnoses by Lesion Category and Proficiency Standard
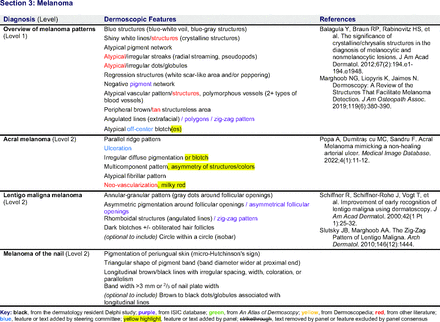
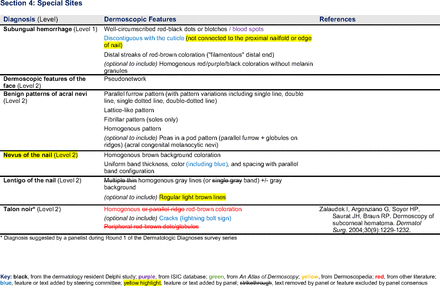
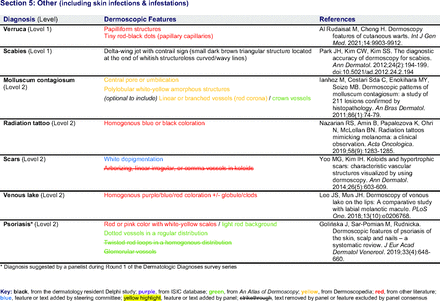
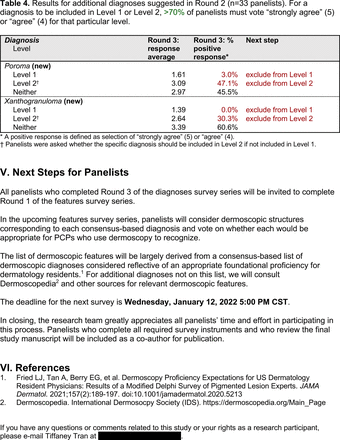
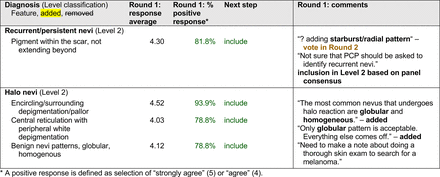
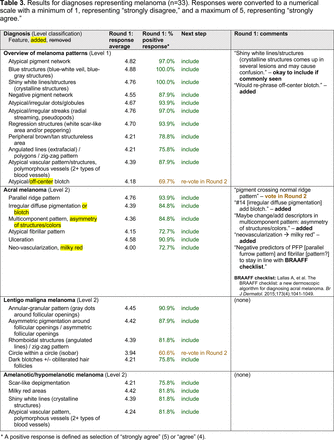
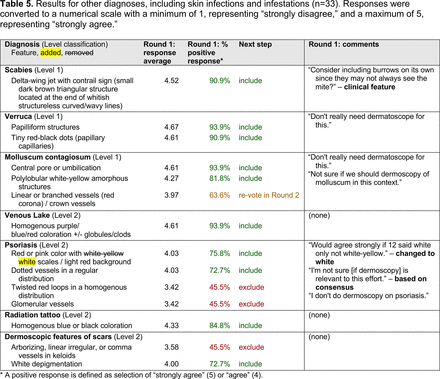
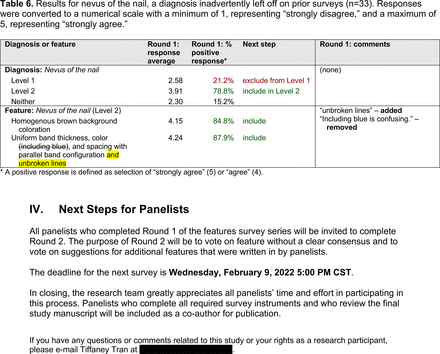
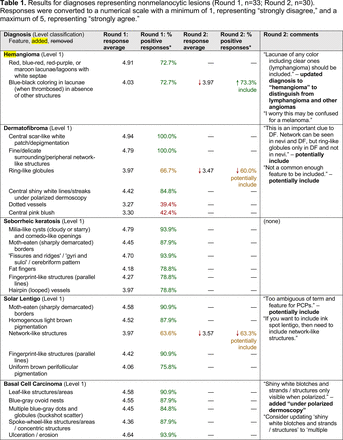
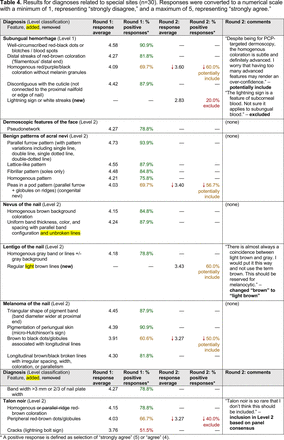
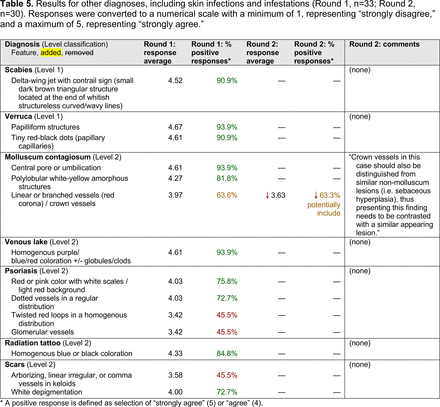
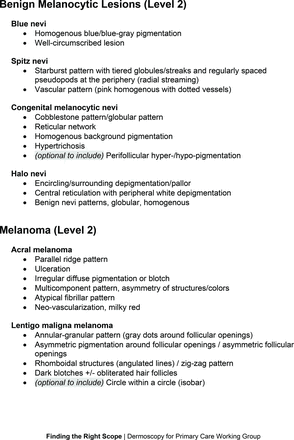
In the features survey series, panelists voted on the inclusion of different dermoscopic features for each included diagnosis. A summary of the features survey results—organized into the categories of nonmelanocytic lesions, benign melanocytic lesions, melanoma, special sites, and other diagnoses—is included in Tables 3-7. Of the 156 total features surveyed, 6 represented additional features written in by panelists, and 120 features received >70% positive responses and reached final consensus for inclusion (62 in level 1 and 58 in level 2). Certain features may have been excluded if they are rarely seen, challenging to discern, and/or of poor diagnostic value. Of note, 19 features (4 in level 1 and 15 in level 2) received >50% but <70% positive responses and thus did not reach final consensus. However, depending on the degree of interest and skill level of the educational cohort, these features may be added as a learning objective at the discretion of the curricular development team.
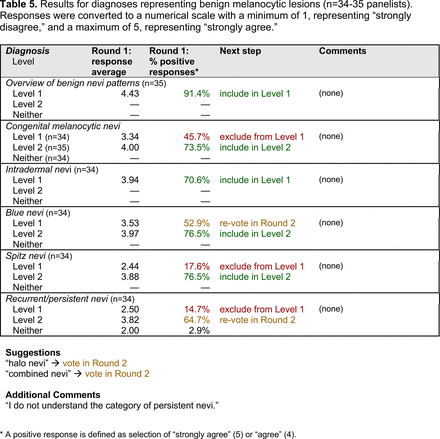
Dermoscopic Characteristics of Nonmelanocytic Lesions
Dermoscopic Characteristics of Benign Melanocytic Lesions
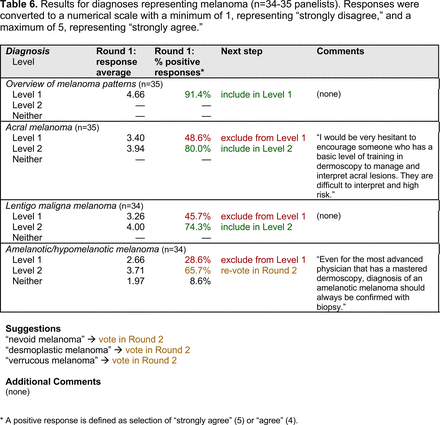
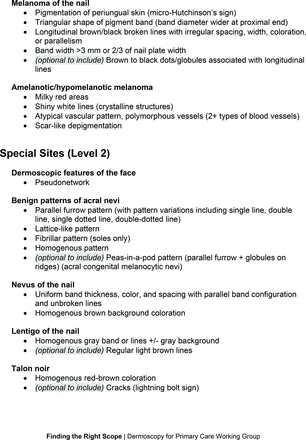
Dermoscopic Characteristics of Melanomas
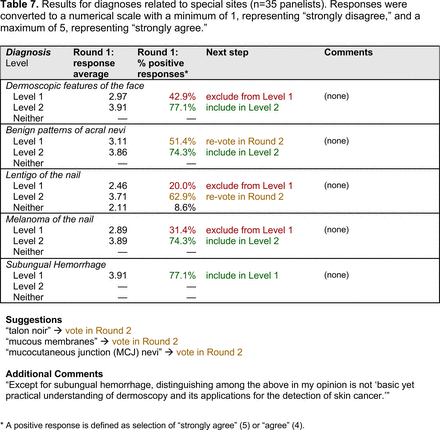
Dermoscopic Characteristics of Benign Diagnoses at Special Sites
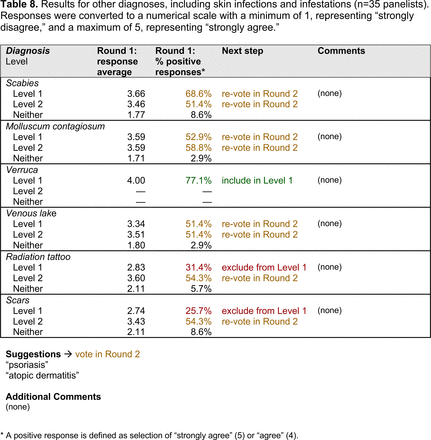
Dermoscopic Characteristics of Other Diagnoses, Including Skin Infections and Infestations
The online appendices contain the final list of diagnoses and their associated features, organized into levels 1 and 2 based on Delphi agreement. For each associated feature, dermoscopy users may customarily refer to different nomenclatures to describe the same pattern. In this study, the exact wording for each feature was considered less important than the described feature itself.
Discussion
Through a modified Delphi exercise, an expert panel that comprised family medicine physicians, internal medicine physicians, and dermatology specialists achieved consensus on proficiency standards for PCPs learning or using dermoscopy. This collaboration between primary care and dermatology reflects a growing national partnership that has been emerging as an important strategy for skin cancer prevention and detection, especially in rural areas.
Given the range of interest in dermoscopy among PCPs, the consensus process generated 2 levels of proficiency standards. The focus of level 1 (foundational proficiency) is training in the basic skills required to differentiate between benign and malignant lesions under dermoscopy. As expected, level 1 teaches an overview of nevi patterns and melanoma patterns as well as classic features for keratinocyte carcinomas, namely BCC and SCC.
Level 1 also contains common benign diagnoses that closely align with the triage amalgamated dermoscopy algorithm (TADA).27,28 This diagnostic aid trains learners to first search for specific features of common benign diagnoses (ie, angioma/hemangioma, seborrheic keratosis, dermatofibroma).29,30 In suspicious lesions, learners next evaluate for characteristic features of malignant diagnoses that would warrant biopsy, excision, or referral to a specialist.31 Training programs based on TADA have been shown to improve the sensitivity for skin cancer detection compared with baseline.29⇓⇓–32 Given the proven effectiveness of TADA in training PCPs and novices,33 PCP-targeted dermoscopy education based on level 1 may begin with TADA and then continue to the other level 1 diagnoses.
Extending beyond level 1, level 2 is intended for more experienced PCPs who desire more advanced dermoscopy skills. Compared with those in level 1, diagnoses in level 2 are mostly considered less common in the general population and/or more challenging to discern (eg, pigmented actinic keratosis, lichen planus-like keratosis). Level 2 also incorporates different types of melanoma (eg, lentigo maligna melanoma, amelanotic/hypomelanotic melanoma) and benign nevi (eg, blue nevi, acral nevi) and demonstrates a broader utility of dermoscopy in the identification of other diagnoses frequently encountered by PCPs (eg, molluscum contagiosum, psoriasis).
With the exception of 1 diagnosis that required 2 rounds of feedback (ie, scabies), all level 1 diagnoses were deemed “foundational” by the panel during the very first round of voting, demonstrating strong consensus on the diagnoses reflective of a basic yet practical skillset for PCPs. Subsequent rounds focused on sorting between level 1 and 2 diagnoses and identifying diagnoses that should be excluded from either level. For instance, the decision-making process for lichen planus-like keratosis required 3 rounds of voting before assigning the diagnosis to level 2.
The outcome of this PCP-focused consensus effort differs in some ways from the proficiency standard developed by the MPWG-PLS for dermatology residents.21 In addition to assigning diagnoses to level 1 or 2, this panel approved the inclusion of additional diagnoses and excluded clear cell acanthoma from either level. Of the 15 total additional diagnoses suggested by panelists, 1 (ie, verruca) reached consensus for inclusion in level 1, and 4 (ie, ink spot lentigo, halo nevi, talon noir, psoriasis) in level 2. The expert focus group also removed simple lentigo from the list due to its overlap with solar lentigo. With the exception of psoriasis (level 2), all other diagnoses excluded from the foundational proficiency standard for dermatology residents (eg, poroma, Merkel cell carcinoma, nevoid melanoma, desmoplastic melanoma) were likewise excluded from the foundational and intermediate proficiency standards for PCPs. The mutual exclusion of these extremely rare and/or challenging diagnoses by this panel serves to validate the results of this consensus process.
This consensus statement will contribute to the development of effective educational interventions that teach expert-approved learning objectives and have content validity.24 It may also serve as the basis of formal proficiency certification or continuing medical education credit for PCPs. Yet, the application of this consensus statement comes with an important caveat: educators and learners alike are strongly discouraged from approaching dermoscopy training as a process akin to the rote memorization of a list of diagnoses and features. Efficient interpretation of dermoscopic images relies heavily on pattern recognition skills34 and “fast thinking.”35 Though the educational science for dermoscopy education remains to be further developed, active learning strategies, such as visual perceptual training36 or deliberate practice,37 are generally more effective than passive instructional approaches. Future studies will explore the application of this consensus statement to dermoscopy educational interventions for PCPs. Further research is also needed to determine best practices for dermoscopy proficiency assessments.
Conclusions
Dermoscopy is a valuable tool that assists clinicians in discriminating malignant from benign skin lesions. For PCPs who treat skin conditions and evaluate skin lesions, dermoscopy training improves sensitivity for skin cancer diagnosis. However, 1 of the obstacles to developing a standardized dermoscopy curriculum for PCPs has been the lack of consensus on appropriate learning objectives. To PCPs using dermoscopy in clinical practice, this study provides meaningful insight into the diagnoses and features that an expert panel considers important to recognize, especially in the course of identifying skin cancer.
The consensus statement generated by this modified Delphi study will inform future dermoscopy training programs designed to support early skin cancer detection by PCPs. Through the dissemination of a standardized dermoscopy curriculum, the dermatoscope may become increasingly recognized as a valuable component of the PCP’s toolbox alongside other commonly used medical instruments such as the ophthalmoscope, otoscope, and stethoscope.38 The ultimate goal of these dermoscopy training initiatives would be to decrease patient morbidity and mortality from skin cancer, especially in regions without convenient access to dermatology specialists.
Acknowledgments
The research team wishes to acknowledge Dr. Lauren Fried for her guidance on the study design.
Appendices.

























































Notes
This article was externally peer reviewed.
Funding: This project was supported in part by the generous philanthropic contributions of the Lyda Hill Foundation to the University of Texas MD Anderson Cancer Center Moon Shots Program. The funding source was not involved in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/36/1/25.full.
- Received for publication April 8, 2022.
- Revision received August 16, 2022.
- Accepted for publication August 17, 2022.






