Abstract
Background: Angioedema of the tongue, oral mucosa, and pharynx is a highly visible and easily diagnoseable side effect of Angiotensin-converting enzyme inhibitors (ACEI). Angioedema of the small bowel is a rarer, and underrecognized, adverse event that may present as a diagnostic challenge due to its nonspecific symptoms and lack of visibility, and because of a general lack of awareness of it among physicians. Failure to consider ACEI-induced angioedema of the small bowel in differential diagnoses may result in unnecessary interventions and delay of treatment.
Methods: We describe the case of a 61-year-old female who was diagnosed with ACEI-induced angioedema of the small intestine after several repeated evaluations. We undertook a literature search to help provide diagnostic, treatment, and management suggestions in patients with ACEI-induced angioedema of the small intestine.
Results and Conclusion: In the literature, we found that age, patient demographics, and careful medical reconciliation, paired with diagnostic clues in radiology, can assist in accurate diagnosis. More broadly, family and emergency medicine physicians, surgeons, radiologists, and internists should be aware of this rare side effect caused by this commonly prescribed medication to avert unnecessary medical treatments and procedures.
- Adverse Drug Event
- Angioedema
- Angiotensin-Converting Enzyme Inhibitors
- Case Report
- Disease Management
- Family Medicine
- Hospitalists
- Hypertension
- Small Intestine
Introduction
Angiotensin-converting enzyme inhibitors (ACEI) reduce morbidity and mortality in hypertensive patients with coronary artery disease, heart failure, and high-risk diabetes. ACEIs have become the most commonly prescribed drug in the United States, with over 40 million people estimated to be receiving therapy.1,2,3As such, there has been an increased prevalence of side effects, including dry cough, dizziness, and headaches. Less commonly, ACEI-induced angioedema may occur, which causes rapid swelling of the tongue, oral mucosa, and pharynx, and requires hospitalization for airway protection.4 In some patients, isolated angioedema of the small intestine has also been observed. ACEI-induced angioedema of the small bowel can be difficult to diagnose, as patients present with nonspecific symptoms such as abdominal pain, diarrhea, nausea, or emesis. The vague nature of presentation often leads to misdiagnosis and may result in unnecessary ED visits, frequent hospitalizations, and invasive procedures—including endoscopy, intestinal biopsy, diagnostic laparoscopy, and in some cases, exploratory laparotomy with small bowel resection.3⇓–5
Recurrence of angioedema of the small intestine is likely if the ACEI is not recognized as the offending agent and promptly stopped by the clinician. We present a case of a patient diagnosed with ACEI-induced angioedema of the small intestine, the treatment and hospital course, and the pathophysiology.
Case Presentation
A 61-year-old female with a past medical history of asthma, seasonal allergies, and hypertension presented to the ED with severe, 10/10 diffuse episodic abdominal pain. The pain originally began 2 months before her ED visit, worsened after eating and alleviated with supination. Associated symptoms included nausea, cough, and occasionally vomiting. The patient’s review of systems was negative for blood in her emesis or stool, fever, chills, chest pain, or shortness of breath. Medications before the presentation included albuterol nebulizer as needed, atorvastatin 40 mg daily, and lisinopril 20 mg daily. She had presented to both her primary care doctor and the ED 1 month prior with similar symptoms. At that time, her metabolic panel and liver function test were normal, and she was sent home after receiving IV fluids.
On return to the ED, her vital signs were stable. Physical examination was benign except for mildly tender abdomen in all 4 quadrants with guarding. Her complete blood work was remarkable only for a white blood cell count of 12.20 thousands/uL (4.31 - 10.16 thousand/uL) with neutrophil predominance of 68%.
CT scan of the abdomen and pelvis revealed submucosal edema of the jejunum and engorgement of the mesenteric vessels concerning for enteritis. The case was discussed with the diagnostic radiologist, who proposed ACEI-induced angioedema given the patient’s lisinopril use. The surgeon on call agreed with this assessment and the decision was made to proceed with conservative management. The patient’s lisinopril medication was held, she was made NPO with bowel rest, and her pain was managed with morphine and acetaminophen.
The following day, the patient’s symptoms had completely resolved and her abdominal examination was benign. Her Lisinopril was switched to hydrochlorothiazide. She was discharged from the hospital and followed up in the clinic a week later and remained symptom-free. Her allergy list in her electronic medical record was updated to include ACEI.
Discussion
Angioedema is a pale, nonpruritic, well-demarcated, nonpitting edema involving the skin and subcutaneous tissue or submucosa.6 Angioedema occurs in 0.1 to 2.2% of patients treated with ACEI. Although it is easily recognizable when it involves a patient's face, tongue, pharynx or larynx, isolated involvement of the small bowel can be a diagnostic dilemma.4,11
ACE inhibitors reduce blood pressure by inhibiting the conversion of angiotensin I to angiotensin II, which is a potent vasoconstrictor. Angiotensin II is also responsible for the catabolism of the nonapeptides bradykinin and substance P, which increase vascular permeability and plasma extravasation into the submucosa tissue.6 While a decrease in circulating angiotensin II reduces systemic vasoconstriction, it can also result in an increase in circulating substance P and bradykinin, leading to angioedema.
In addition to angiotensin II, enzymes aminopeptidase P (APP) and dipeptidyl peptidase IV (DPP-IV) have also been found to participate in the breakdown of bradykinin and substance P. Case-controlled studies found decreased activities of DPP-IV and APP in patients with ACEI angioedema.7 Further analysis of the enalapril group of the OCTAVE study identified 4 groups of patients who had a statistically significant higher incidence of angioedema: patients aged 65 years and over, African Americans, patients with a history of drug rash, and patients with seasonal allergies.6 Women and smokers were also found to have a higher risk of developing angioedema. The patient described in this report was both female and a smoker.
It was found that estrogen induces the expression of bradykinin, suggesting the differences found between men and women. Though the exact cause of angioedema in African Americans is uncertain, Brown et al proposed that they might have a higher sensitivity to bradykinin. Findings also confirmed that patients over the age of 65 had decreased levels of DPP-IV, explaining the increased prevalence in that population.6,11 Knowledge of the elevated risk in select populations can help guide future diagnoses.
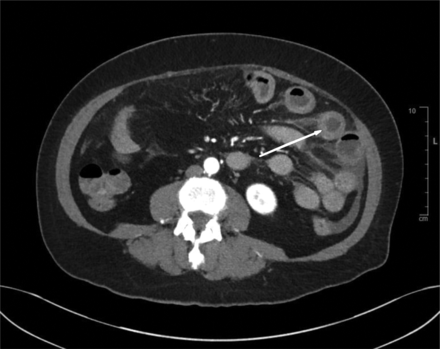
Differential diagnoses based on history, presentation, and labs include infectious colitis, vasculitis, intramural hemorrhage, appendicitis, hepatobiliary disease, SMA syndrome, small bowel ischemia, and Crohn's disease.6 The wall edema and inflammation of the long segment of the jejunum are both common in patients with ACEI-induced angioedema.6 Other common CT findings include ascites, preservation of luminal transit despite thickening, dilatation, and straightening of the small bowel (Figure 1). Many cases involve continuous segments of the small bowel. The affected small bowel typically demonstrated what is known as “target sign.”6 The target sign is produced by alternating enhancement of the mucosa, relatively hypodense submucosa, and enhancing serosa (Figure 2). It is important to note that even with these imaging findings alone, vasculitis, intramural hemorrhage, and small bowel ischemia cannot be ruled out.8 Therefore, it is important to correlate imaging findings with the appropriate symptoms and careful history taking.
Coronal view of patient’s abdominal CT with IV contrast illustrated above shows long segment bowel edema with evidence of engorgement of the mesenteric vessels.
Axial view of patient’s CT abdomen with IV contrast illustrates the classic ‘target lesion’ which depicts alternating enhancement of the mucosa and submucosa.
Recommended treatment for ACEI-induced angioedema is simply to discontinue the medication. Symptoms usually resolve within 24 to 72 hours, so patients should be observed in the hospital setting for at least 24 hours. Adjunct treatment includes antihistamines, subcutaneous epinephrine, and steroids, although controlled studies have not demonstrated efficacy or shortened duration.9,12
Patients who develop ACEI-induced angioedema should never be prescribed ACEI therapy and should list the medication as an allergy. Other classes of antihypertensive medication should be considered for these patients. For patients on ACEI due to comorbid conditions such as diabetes and heart failure, angiotensin receptor blockers (ARBs) can be used as an alternative therapy.10 This class of medication acts directly on the angiotensin receptor, inhibiting the binding of the potent vasoconstrictor angiotensin II.10 The inhibitory effect on vasoconstriction is still achieved without changing the levels of angiotensin II. Based on the low prevalence of cross-reactivity found in the literature, the National Kidney Foundation, the American College of Cardiology, and the American Heart Association recommended ARBs as an alternative in the treatment of heart failure, acute myocardial infarction, and secondary prevention of cardiovascular disease.10,12 Risks and benefits should always be discussed with the patient and monitoring will be subsequently required.
Conclusion
ACEI-induced angioedema of the intestine is a rare occurrence, though perhaps underreported due to difficulty in recognition and lack of awareness among clinicians. In patients taking ACEI who present with abdominal pain and CT findings suggestive of small bowel edema, clinicians should have a high index of suspicion for ACEI-induced angioedema. Early cessation of ACEI is curative while also averting unnecessary medical treatment and surgical intervention. ACEI therapy should never be reinitiated in patients who develop this reaction. ARBs should be considered as an alternative agent in patients with heart failure, acute coronary heart disease, and diabetes.
Notes
This article was externally peer reviewed.
Funding: None.
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/36/1/160.full.
- Received for publication August 10, 2022.
- Revision received October 24, 2022.
- Accepted for publication October 26, 2022.