Abstract
Introduction: There has been an increasing focus on improving value in health care and deimplementing the use of low-value services, such as prostate cancer (PC) screening for men aged >70 years. The objectives of this study are to (1) identify the proportion of primary care visits at which low-value PC screening is ordered, and (2) identify predisposing, enabling, and health care need characteristics associated with low-value PC screening in the United States.
Methods: This was a secondary analysis of the National Ambulatory Medicare Care Survey datasets from 2013 to 2016 and 2018. Andersen’s Behavioral Model of Health Services Use guided independent variable selection. Weighted multivariable logit models were used to analyze data.
Results: There were 6.71 low-value prostate-specific antigens (PSAs) per 100 visits and 1.65 low-value digital rectal exams (DREs) per 100 visits. For each additional service ordered by primary care providers, the odds of ordering a low-value PSA increased by 49%, and the odds of performing a low-value DRE increased by 37%.
Conclusions: The use of low-value PSAs and DREs was sizable during the observed time period. Organizations who want to reduce low-value PSAs and DREs may want to focus interventions on providers who order a high number of tests.
- Early Detection of Cancer
- Geriatrics
- Logistic Models
- Men's Health
- NAMCS
- Physicians
- Primary Health Care
- Prostate Cancer
- Prostate-Specific Antigen
Introduction
Prostate cancer (PC) is the most common nonskin cancer in American men and the second-leading cause of cancer-death.1 The American Cancer Society estimates that in 2021, 248,530 men will be diagnosed and more than 34,000 men will die from PC.1 In 2010, the direct costs of PC were estimated to exceed more than $19 billion by 2020.2 Men who are at the highest risk of being diagnosed with PC are older men and non-Hispanic Black men.3
Recommendations for PC screening are heterogeneous and have been constantly evolving, potentially confusing providers.4 Although some forms of PC are aggressive, most PC is slow growing and may never cause symptoms. This heterogeneity in aggressiveness is what makes PC screening so complex; PC-related death is a significant cause of mortality in men, and it is important to identify the aggressive forms at the right time while not overtreating indolent PC. Currently, it is thought that PC screening confers the most benefit between the ages of 55 to 69 years with the lowest risk of overdiagnosis and overtreatment.5 Reducing overdiagnosis and overtreatment of indolent PC is important to improve the value of health care for men. Men who are over 70 years of age have a much higher risk of experiencing overdiagnosis and overtreatment for a cancer that may be indolent and would not experience an improvement in mortality.5 The United States Preventive Services Task Force (USPSTF) 2018 prostate cancer screening recommends against providing men 70 years and older with prostate-specific antigen (PSA)-based testing for PC screening.5 The American Urological Association has recommended against PSA-based PC screening for men over the age of 70 years of age since at least 2013.6
The Center for Value-Based Insurance Design defines low-value care as: “services that provide little or no benefit to patients, have potential to cause harm, incur unnecessary cost[s]…or waste limited health care resources.”7 Screening for PC in men over 70 years may be an example of “low-value care” because screening may introduce unnecessary risks without increasing the lifespan. This low-value care is common among men over the age of 70 years, despite guidelines recommending against this screening.8⇓⇓⇓–12 Further, there has been an increasing focus on reducing low-value care while improving quality of care.7 Most prior research on low-value PC screening has been conducted among commercially insured men, veterans in the Veterans Administration health care system, and single health care systems.8⇓⇓–11 These analyses, however, are not nationally representative of clinic visits or may not include men who are uninsured or who are covered through Medicaid or Medicare.
The National Ambulatory Medical Care Survey (NAMCS) is a nationally representative dataset that is conducted yearly at nonfederal physician clinics.13 This dataset presents a unique way of examining the prevalence of low-value PC screening nationally. The objectives of this study are to (1) identify the proportion of preventive visits at which low-value PC screening is ordered by primary care clinics, and (2) identify patient, visit, and provider characteristics that are associated with low-value PC screening.
Methods
Participants and Procedure
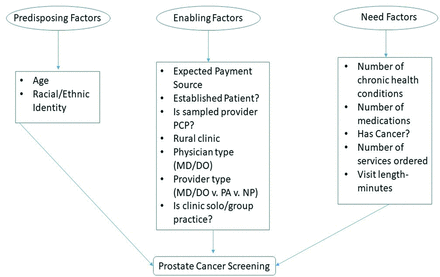
This study was approved by the institution’s institutional review board. We used and combined the 2013 to 2016 and 2018 NAMCS datasets. The 2017 NAMCS datasets are not yet available. The NAMCS datasets and the methodology used to create the datasets are well characterized.13⇓–15 Briefly, the NAMCS datasets are at the level of the clinic visit and are conducted annually by the National Center for Health Statistics (NCHS) at nonfederal physician’s offices. We restricted the dataset to: (1) visits where the patient was male, (2) the age of the patient was 70 and over, (3) the visit occurred at a primary care clinic, and (4) the major reason for the visit was for a (a) new problem, (b) chronic problem, or (c) preventive visit. Using a priori and observed diagnosis codes (Appendix), we excluded specific diagnosis codes that could be indicative of a diagnostic PSA/digital rectal exam (DRE) instead of a screening PSA/DRE using the primary diagnosis recorded, which is indicative of the principal reason for the visit.16,17 Examples of diagnosis codes that we excluded were: (1) malignant neoplasm of prostate, (2) urinary incontinence, (3) unspecified disorder of prostate, and so forth. We also excluded visits in which the primary care variable was blank and where the solo practice variable was blank, unknown, or refused. We used the physician survey variables, PSA and RECTAL, to identify whether a PSA and/or DRE was ordered/provided. Briefly, census field representatives completed an abstraction tool from medical charts using a secure web portal or physicians (or their representatives) completed the abstraction tool. Almost all NAMCS data (>99% for each year 2013 to 2015, all data in 2016 and 2018) were collected by census field representatives. In-depth explanations and coding for each NAMCS variable can be found online.15 Andersen’s Behavioral Model of Health Services Use (ABM) guided selection of variables (Figure 1).18 A priori, we operationalized each construct of the ABM, then examined the NAMCS documentation to identify and select only those variables for analysis.
Operationalization of Andersen’s Behavioral Model of Health Services Use. Abbreviations: PCP, primary care provider; PA, physician assistant; NP, nurse practitioner.
Statistical Analysis
All analyses were conducted using SAS v9.4 (Cary, NC). First, we described the visits with low-value PSA and DRE characteristics using appropriate descriptive statistics for each year separately and combined. We examined weighted bivariate relationships between receiving a PSA and a DRE separately with patient (eg, source of payment, race/ethnicity), visit (eg, number of tests ordered, time spent with physician, year of visit), and provider characteristics (eg, PA included in visit [Yes vs No], NP included in visit [Yes vs No]) using appropriate tests (PROC SURVEYFREQ, PROC SURVEYMEANS, PROC SURVEYREG) for complex survey designs. To ensure accuracy regarding race and ethnicity variables, we only used the imputed race/ethnicity variable. We only examined private insurance, Medicare, and Medicaid source of payment variables. There were no DREs for patients whose expected source of payment was Medicaid, self-pay, or “Other,” therefore we only examined visits in which the expected source of payment was private insurance or Medicare for the DRE outcome. We then constructed weighted multivariable logit models (PROC SURVEYLOGISTIC) using the statistically significant variables from the bivariate tests (at the α < 0.05 level) for both PSA and DRE. The weighted logit regression models were 2-sided with an α < 0.005 to indicate statistical significance to reduce the risk of a type I error.19 Results from the weighted logit models are presented using odds ratios (ORs) and 99.5% CIs.
Results
The results indicate that there were 6.71 low-value PSAs per 100 visits (95% CI, 5.19-8.23, weighted = 8162,325, unweighted, n = 202) and 1.65 low-value DREs (95% CI, 0.91-2.38, weighted = 2002,919, unweighted n = 53) that occurred between 2013 to 2016 and 2018. Figure 2 presents the prevalence rate of low-value PSAs and DREs per 100 visits by year. Table 1 presents the descriptive characteristics of the sample.
Use of low-value prostate-specific antigen and digital rectal exam in primary care clinics during 2013 to 2018. Abbreviations: PSA, prostate-specific antigen; DRE, digital rectal exam; CI, confidence interval.
Patient, Provider, and Visit Characteristics of National Ambulatory Medical Care Survey Sample (Unweighted n = 2,964, Weighted n = 121,633,833)
According to the weighted logit regression model (Table 2), visits where there were a higher number of services ordered/provided were significantly more likely to receive a low-value PSA (OR = 1.49, 99.5% CI, 1.33, 1.67) and more likely to receive a low-value DRE (OR = 1.37, 99.5% CI, 1.15, 1.63). Visits in which the patient had more previous visits was less likely to receive a low-value DRE (OR = 0.92, 95% CI, 0.85-0.996). The variables that were included in the multivariable weighted logit regression models were all significant in bivariate tests (P < .05).
Multivariable Logistic Regression Results Predicting Low-Value Prostate-Specific Antigen Blood Test and Digital Rectal Exam (Unweighted n = 2,964, Weighted n = 121,633,833)
Discussion
Using nationally representative data, we found sizable rates of low-value PSA (6.71/100 visits) and low-value DRE (1.65/100) and that incidence of low-value PSA and DRE was related to the number of services provided/ordered by the physician. Specifically, for each service ordered, there was a 49% increase in the odds of a low-value PSA and a 37% increase in the odds of a low-value DRE. Number of previous visits was also found to be related to receiving a low-value DRE; for each additional previous visit, there was a decrease in the odds of receiving a low-value DRE. We also found low-value PSA declined after 2014 while no trend was identified for DRE in primary care visits.20⇓–22 This declining prevalence of PSA screening was consistent with reports studying PSA screening using Medicare data, which also found decreasing PSA screening in men over 70 over a similar time period.9,20 Our findings build on and extend previous research using NAMCS datasets from 2005 to 2012.21
The “shotgun” approach to medical testing, where a provider orders all of the possible tests during a medical visit, is a well-known phenomenon and may cause the wasting of scarce resources at best and at worst subjects the patient to potentially harmful tests and procedures afterward.23,24 Patient preferences for screening for PC have been shown to be highly preference sensitive, and patients have been shown to be willing to undergo PC screening even though there are potentially serious risks to avoid PC death.25 Providers may use the “shotgun” approach to ensure they do not miss a potential problem and/or to avoid malpractice litigation.23,26,27 Alternatively, providers who ordered these tests may simply be responding to patient requests. Therefore, our findings suggest that providers who order a lot of tests may be more likely to order low-value tests, for example, low-value PSA and low-value DRE. Medical educators and health care organizations who wish to reduce the use of low-value services should focus interventions (eg, audit and feedback) on providers who order a lot of tests.
Prior research has shown that the use of PSA and DRE has declined over time and has coincided with guidelines’ screening recommendations.22,28⇓–30 In 2012, the USPSTF recommended against any PSA-based screening in men but then changed their recommendation in 2018 to only not screen men who were 70 years and older. Previous studies have relied on commercially insured men or patient-reported rates of PSA testing.29,30 We believe the findings of this study are important because we used a nationally representative clinical dataset that includes men who are uninsured or insured through traditional Medicare.
While the findings of our study are important, there are limitations that affect generalizability. First, we only examined primary care PC screening, so we did not include urologists’ PC screening behaviors. Second, the results of this study may not be generalizable to all practices and all types of low-value services. Third, the absence of DRE in 2018 suggests this finding may be related to the large year-over-year decline in number of sampled clinic visits rather than an actual absence of DRE screening in primary care. As discussed above, DRE was relatively rare in 2012, and the number of NAMCS datasets was 72% smaller in 2016 and 2018 compared with 2014, which may mean the NAMCS sampling design could not identify those visits in which DRE occurred. Any data after 2015 needs to be interpreted with caution based on small sample sizes; NCHS recommends that minimum number of 30 observations and a relative standard error of less than 30% to ensure reliability of estimates.31 The likelihood of this impacting our findings is low because we combined multiple years’ data as recommended by NCHS. The decline in sampled visits could also be related to the general decline in primary care visits nationwide.32
Despite the limitations noted, this study contributes to what is known about PC screening in the United States. We found that low-value PC screening continues to be used in primary care visits. We found that a higher number of services ordered/provided increased the odds of low-value PSA and low-value DRE. Our study confirms and extends previous findings about the use of low-value prostate cancer screening in older men.
Appendix. Excluded diagnosis codes that suggest diagnostic prostate-specific antigen and digital rectal exam
Notes
This article was externally peer reviewed.
Funding: None.
Conflict of interest: The authors report no conflicts of interest.
To see this article online, please go to: http://jabfm.org/content/36/1/152.full.
- Received for publication May 18, 2022.
- Revision received August 22, 2022.
- Accepted for publication August 24, 2022.