Abstract
Purpose: Prediabetes is a serious public health concern, with 34.5% of US adults meeting the criteria for prediabetes. The American Diabetes Association has highlighted metformin therapy as a consideration for individuals with BMI ≥ 35 kg/m2, those aged < 60 years, and women with a history of gestational diabetes. We examined metformin prescription rates among a national sample of commercially insured, higher risk patients with prediabetes.
Methods: We gathered 2012 to 2018 demographic, laboratory, and prescription data for 53,551 patients with prediabetes from the IBM MarketScan research database. Our primary outcome was metformin prescription rates 1 or 3 years after a laboratory confirmation of prediabetes among patients who have a BMI ≥ 35 kg/m2 or are aged < 60 years.
Results: Overall, 2.4% (n = 1,124) of patients received a metformin prescription within 1 year of a laboratory confirmed prediabetes result, including 2.4% of patients aged < 60 years and 10.4% of those with BMI ≥ 35 kg/m2. By a 3 year follow-up, 4.1% (n = 1901) received a metformin prescription, including 3.9% of patients aged < 60 years and 14.0% with BMI ≥ 35 kg/m2. Patients who developed type 2 diabetes within the 1 (n = 2,769) or 3 year (n = 7,268) follow-up periods were excluded from analysis.
Conclusions: Few prediabetes patients who were either obese or aged < 60 years received a metformin prescription between 2012 and 2018. Prescription rates increased slightly between 1 and 3 years after a prediabetes diagnosis, so strategies to support timely intervention among higher risk patients with prediabetes are critically needed.
- Blood Glucose
- Body Mass Index
- Chronic Disease
- Fasting
- Follow-Up Studies
- HbA1c
- Metformin
- Obesity
- Pharmaceutical Preparations
- Population Health
- Prediabetes
- Prescriptions
- Type 2 Diabetes Mellitus
Introduction
Diabetes is a serious public health concern, with 10.2% of the US adult population diagnosed and an additional 2.8% undiagnosed with the condition.1 In 2017, diabetes ranked as the seventh leading cause of death the US, with total direct and indirect costs of $327 billion.1 Type 2 diabetes is often preceded by prediabetes, characterized as blood glucose levels that are elevated above normal, but not high enough to be classified as diabetes. Adults with prediabetes are at an increased risk of developing type 2 diabetes. In the US, 88 million adults (34.5%) currently have prediabetes,1 which is approximately 1 in 3 individuals. Evidence suggests that delaying or preventing the transition to type 2 diabetes may have implications for life expectancy and quality of life.2⇓–4 Type 2 diabetes conversion rates vary depending on the individual and situation, with national prospective cohort studies showing conversion ranging from 6 to 11% annually.5,6 Studies have shown diabetes incidence can be reduced through effective preventive interventions, such as participation in intensive lifestyle change programs (LCP) like the National Diabetes Prevention Program (National DPP) lifestyle change program, or with pharmacotherapy such as metformin.7⇓⇓⇓⇓–12 Unfortunately, referral and uptake of interventions to prevent or delay type 2 diabetes remain low.
Although national clinical recommendations identify metformin therapy as an effective preventive intervention,13,14 there is no Food and Drug Administration (FDA) indication for metformin use for diabetes prevention and treatment effects may vary depending on risk levels of the individual.9 Clinical recommendations highlight metformin use for 3 higher risk groups: individuals with BMI ≥35 kg/m2, those aged < 60 years, and women with a history of gestational diabetes where it has been shown comparably effective to lifestyle modification.14 In addition to potential for type 2 diabetes risk reduction, metformin therapy is also a feasible strategy in real world settings, given its association with cost savings.15 Thus, our goal was to focus on metformin prescription rates for the 3 groups identified as most likely to benefit. In this report, we examine rates of metformin prescription among a national sample of commercially insured US patients with a BMI ≥35 kg/m2 or aged < 60 years.
Methods
We conducted a retrospective analysis of deidentified data from a national cohort of commercially insured patients derived from a database containing electronic health record (EHR) and claims data including, outpatient services, inpatient services, laboratory values, and prescription drugs, for approximately 4.1 million commercially insured US patients from the 2012 to 2018 IBM MarketScan research database. Variables extracted from these files included age, sex, results for glycemic laboratory tests, body mass index (BMI), diagnosis codes, and a dichotomous indicator for receipt of a metformin prescription.
Our study included patients aged 18 years and older with at least 1 laboratory confirmed result in the prediabetes range: fasting plasma glucose (FPG: 100 to 125 mg/dL) or glycohemoglobin A1c (HbA1c: 5.7-6.4%). Laboratory results were excluded when a prior primary or secondary diagnosis of type 2 diabetes was indicated by an International Classification of Diseases, Tenth Revision (ICD-10) code with a prefix of E11 and were extracted from outpatient service claims to indicate a history of diabetes before a laboratory result. Laboratory results indicating a type 2 diabetes diagnosis within specified follow-up periods (1 or 3 years) were excluded from analysis. Patients with a history of end stage renal disease (ESRD) based on ICD-10 code N18.6, and patients currently pregnant, ICD-10 code with prefix of Z33, were also excluded. Finally, women with a history of gestational diabetes were excluded from analysis due to very low sample sizes.
We obtained BMI values within 1 year of a patient's qualifying laboratory result in the prediabetes range date, with the nearest date selected when multiple values were in that period. Date of metformin prescriptions after a laboratory result were classified into 2 groups: within 1 year or within 3 years. Indications of a metformin prescription within these groups were aggregated at the patient level (Figure 1).
Study flow diagram. Abbreviations: FPG, fasting plasma glucose; HbA1c, glycohemoglobin A1c.
Results
Among the 139,274 patients with available laboratory result(s), 53,551 (38.4%) had at least 1 laboratory result in the prediabetes range and a mean age of 58.9 years. Approximately 57% (n = 30,472) of the study population met the American Diabetes Association's (ADA) higher risk criteria for 1 of the subgroups: 14.9% (n = 7963) BMI criteria for metformin therapy (35 ≥ kg/m2) and 51.5% (n = 27,582) were aged < 60 years (with no comorbidities). No BMI values were recorded for 45.4% (n = 24,230) of the population. Progression from prediabetes to type 2 diabetes within 1 year or 3 years of initial laboratory result occurred in 5.2% and 13.5% of patients, respectively (Table 1).
Characteristics of Patients with at Least One Laboratory Result in Prediabetes Range
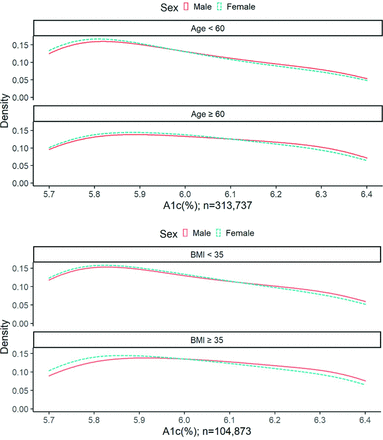
Overall, 1214 patients (n = 50,782; 2.4%) received a metformin prescription in the first year after a laboratory confirmation of prediabetes and 1901 patients (n = 46,283; 4.1%) received a metformin prescription within 3 years (Table 2). Rates of metformin prescriptions 1 year after a prediabetes laboratory confirmation varied by ADA risk subgroup; 2.4% of those aged < 60 years and 10.4% of those with a BMI ≥ 35 kg/m2 received a prescription. When the window for metformin prescription was increased to 3 years, rates of metformin therapy increased to 3.9% for those aged < 60 years and 14.0% for those with BMI ≥ 35 kg/m2. For patients who met both criteria (aged < 60 years and BMI ≥ 35 kg/m2), metformin prescription rates were 10.5% within the first year and 13.8% within 3 years. Kernel density plots demonstrate varying distributions of laboratory results between males and females who meet ADA-criteria for metformin therapy (Figures 2 and 3).
Density plots for glycohemoglobin (HA1c) laboratory results in prediabetes range, with American Diabetes Association (ADA) criteria. *Body mass index (BMI) panel includes only patients with most recent BMI measurement ± 365 days from time of first glycohemoglobin A1c (HbA1c) laboratory result.
Density plots for Fasting Plasma Glucose (FPG) laboratory results in prediabetes range, with American Diabetes Association (ADA) criteria. *Body mass index (BMI) panel includes only patients with most recent BMI measurement ± 365 days from time of first FPG laboratory result.
Proportion of Patients with Prediabetes Receiving a Metformin Prescription, Based on American Diabetes Association (ADA) Criteria (n = 53,551)
Discussion
Our study finds very low rates of metformin prescribing after a laboratory documentation of prediabetes among a large national sample of commercially insured patients. Even among groups recommended for consideration of metformin treatment, about 1 in 50 patients aged < 60 years (2.4%) and 1 in 10 patients with BMI ≥ 35 kg/m2 received a metformin prescription (10.4%) between 2012 and 2018.
Clinical recommendations for prediabetes treatment specify that patients with prediabetes should be offered effective prevention interventions to prevent or delay progression to type 2 diabetes. Our results demonstrate the lack of utilization of 1 such prevention intervention of metformin therapy. Previous studies examining metformin prescriptions have also found low prescribing rates. Our study shows this practice has changed very little in real-world settings up to 2018.12,16 Since the annual progression rate from prediabetes to type 2 diabetes can be as high as 11% in certain populations, these delays represent missed opportunities to delay or prevent progression to type 2 diabetes among adults who are at higher risk.5,6
The low prescription rates found in our study may be due to barriers encountered during clinical encounters, such as limited time with patients and challenges of managing multiple existing chronic conditions. Additional barriers may include a lack of familiarity of the clinical recommendations among prescribers, a lack of FDA approval for metformin for prediabetes, a lack of experience or comfort counseling patients about prediabetes treatment options, patient preference for lifestyle change over pharmacotherapy for prediabetes, and prescriber concerns about patients' medication adherence or potential adverse effects. After extending the time frame for metformin prescription from 1 to 3 years after prediabetes lab confirmation, we found small increases in prescriptions, from 1.5 to 3.6 percentage points. This suggests that adults with prediabetes may be more likely to receive a metformin prescription as the duration of their diagnosis increases and that prescribers may be willing to initiate metformin therapy for prediabetes among specific patients.
There are several limitations in this study. Approximately 45% of patients did not have a BMI value recorded within 1 year of their initial laboratory value, limiting our ability to examine metformin prescription rates for those meeting the ADA criteria of BMI ≥ 35 kg/m2. Our study was only focused on medication prescribing patterns, rather than adherence to the prescriptions. Furthermore, prediabetes is determined only by an HbA1c or FPG lab test and does not consider oral glucose tolerance tests. We were unable to ascertain participation in lifestyle change programs, such as the National Diabetes Prevention Program. Prevalence of prediabetes in this population may have been overestimated, given the criteria of a single abnormal lab value in the follow-up periods. In addition, we were unable to determine metformin prescription rates among women with a history of gestational diabetes, due to the low sample size. Given the current lack of FDA approval for metformin treatment in patients with prediabetes, this may be a barrier to physician prescriptions. Finally, the claims database used may not fully encompass a patient's medical history, with respect to laboratory values, chronic disease, or anthropometric measurements.
Conclusion
Our findings show that, despite the strong body of evidence and recommendations indicating the benefits of metformin therapy for certain adults with prediabetes, prescribing rates remain low. The most recent US Preventive Services Task Force Recommendation Statement and updated Evidence Review have added metformin among diabetes prevention interventions.13,17,18 These changes may help overcome some of these prescribing barriers. Future research and interventions may inform understanding and implementation of comprehensive care management strategies to curb the transition from prediabetes to type 2 diabetes in the US
Notes
This article was externally peer reviewed.
Conflicts of interest: None. The findings and conclusions of this report are those of the authors and do not represent the official position of the American Medical Association.
Funding: Dr. Moin receives support from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (R01DK124503, R01DK127733, and R18DK122372), NIDDK/Centers for Disease Control and Prevention (U18DP006535), and the U.S. Department of Veterans Affairs (QUE20-028 and CSP2002).
To see this article online, please go to: http://jabfm.org/content/35/4/821.full.
- Received for publication December 2, 2021.
- Revision received March 21, 2022.
- Accepted for publication March 25, 2022.