Abstract
This study aimed to help determine the effect of dietary supplements on symptom course and quality of life in patients with mild-to-moderate COVID-19 infection.
Design: We modified the Wisconsin Upper Respiratory Symptom Survey (WURSS) to conduct a 3 arm, parallel, randomized, double-blind, placebo-controlled trial, enrolling patients with mild-to-moderate symptoms of COVID-19 infection. Patients took placebo (n = 34), vitamin C 1000 mg (n = 32), or melatonin 10 mg (n = 32) orally for 14 days.
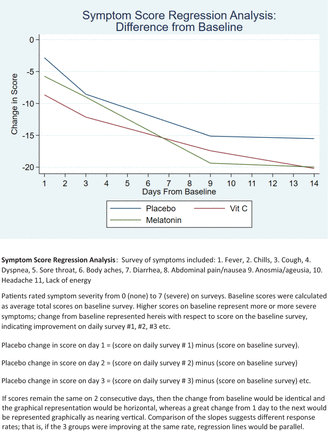
Outcomes: Ninety Eight (98 out of 104 recruited; mean age = 52 years) patients completed the study. Outcomes were calculated as differences from baseline scores on each of 2 WURSS-derived surveys and analyzed using a spline regression analysis. Regarding symptom progression, those patients taking placebo and vitamin C progressed at the same rate. When compared with those taking placebo (coefficient = -1.09 (95% confidence interval [CI] = -1.39 to -0.8) the group taking melatonin had a faster resolution of symptoms (coefficient = -0.63 [95% CI -1.02 to -0.21] P = .003). By day 14 all 3 groups had reached plateau.
Quality-of-life impact analysis demonstrated that the group taking vitamin C improved at the same rate as the group taking placebo (coefficient = -0.71 (95% CI = -1.11 to -0.3)). The group taking melatonin (coefficient = -1.16 (95% CI = -1.75 to - 0.57) P < .005) had a faster improvement in quality-of-life. By day 14 all 3 groups had reached plateau.
Conclusion: Vitamin C 1000 mg once daily has no effect on disease progression. Melatonin 10 mg daily may have a statistically significant effect but it is unclear if this represents a clinically significant benefit to those with mild-to-moderate symptoms of COVID-19 infection. Further study is warranted.
- Ascorbic Acid
- COVID-19
- Dietary Supplements
- Double-Blind Method
- Melatonin
- Quality of Life
- Regression Analysis
- Vitamin C
- Vitamins
- WURSS
Introduction
Severe acute respiratory syndrome coronavirus 2(SARS-CoV-2) emerged in 2019 and has caused an international pandemic of infection known as coronavirus disease of 2019 (COVID-19). COVID-19 infection creates oxidative stress and in severe cases can cause an exponential cytokine cascade resulting in cellular dysfunction and ultimately end organ damage.1 We speculated based on clinical experience that patients diagnosed with this infection might take nutritional supplements despite limited evidence to support their use. Vitamin C (ascorbic acid) and melatonin (N-acetyl-5-methoxytryptamine) have been suggested as potential therapies to protect against the acute lung injury and other sequelae characteristic of COVID-19 infection.2⇓–4 These antioxidizing agents may disrupt the cytokine cascade to prevent further end organ damage in the setting of acute infection.
There is inconclusive support for the use of Vitamin C in the treatment of respiratory disease. An animal study demonstrated that mice with vitamin C deficiency have impaired immune response associated with higher pathologic lung dysfunction when exposed to influenza H1N1 virus.5 Vitamin C levels decrease in those with acute illness, suggesting possible benefit in supplementation at the time of illness.6
While Cochrane reviews have shown consistent benefit of prophylactic vitamin C on the common cold symptom duration, trials of vitamin C as therapy once patients have the common cold are not definitive.7–8 Regarding its use in COVID-19 infection, 4 out of 5 patients with COVID-19 given a combination of antioxidants, including vitamin C, either orally or by IV subsequently had lower inflammatory markers.9 A separate study of 17 inpatients hospitalized with COVID-19 treated with intravenous vitamin C every 8 hours demonstrated decreased inflammatory markers and oxygen demand.10 This data highlights the need for further controlled trials of vitamin C as a low-risk intervention for infections such as COVID-19.
The age-related decline in endogenous melatonin production may put older adults at greater risk of severe COVID-19 infection.11 Furthermore, melatonin has immunomodulatory effects that may be beneficial in treating COVID-19 infection; for example, melatonin inhibits NFkB activation and NLRP3 inflammasome activation, which are mediators of viral proliferation and survival.2,12 Melatonin also reduces oxidative lung injury and inflammatory cell recruitment during viral infections.13 In a recent study of 74 hospitalized patients with COVID-19 infection in Tehran, Iran, subjects were randomized to standard-of-care versus the addition of melatonin 3 mg 3 times daily for 14 days. There was a shorter time to discharge (8.15 vs 4.65 days) without significant difference in intensive care unit (ICU) admissions or 28-day mortality; there were also lower C-reactive protein levels in the melatonin group.14 Another open-label study of 96 patients demonstrated melatonin improved sleep scores and oxygen levels of ICU patients with COVID-19.15 Melatonin supplementation is available over the counter in the United States and there is interest in melatonin's therapeutic potential for treatment of those infected with COVID-19.16–17 Family physicians would like to know how best to advise patients about use of over the counter supplements during infection with COVID-19.
The Wisconsin Upper Respiratory Symptom Survey (WURSS) is a standardized measure that has been validated for assessing the symptoms and impact of upper respiratory disease; it contains questions elucidating disease course as well as the effect those symptoms have on the patient's ability to perform activities while sick.18⇓–20 It should be noted that modified versions of the WURSS are not validated; however, we reached out to the lead author who granted permission to use a modified version.
Methods
From October 5, 2020, until June 21, 2021, we conducted a three-arm, parallel randomized, double-blind placebo-controlled trial in Lancaster County, Pennsylvania. This study received Institutional Review Board approval at Penn Medicine Lancaster General Health (PMLGH) and all procedures followed were in accordance with the ethical standards of PMLGH and the Helsinki Declaration of 1975 as revised in 1983. Participants were recruited from among patients testing positive for COVID-19 using real-time reverse transcriptase polymerase chain reaction at PMLGH testing sites. Patients were sent automated e-mails through their electronic chart portal and invited to enroll via a webpage describing the study. They were then called by our research coordinator who further screened their charts to determine eligibility. (See Figure 1)
Patients were eligible for inclusion in the study if they met the following criteria: experienced fewer than 6 days of infection-related symptoms; English or Spanish speaking; greater than or equal to 40 years of age; ability to use e-mail; and had a primary care physician within the PMLGH system. Patients were excluded if they were hospitalized, incarcerated, or taking melatonin, vitamin C (greater than 100 mg daily) or warfarin. Patients were sent the study description electronically and if interested, were consented over the phone and through e-mail, allowing the research coordinator to collect digital signatures. Once consented, participant e-mail accounts were set up to receive automatic REDCap surveys that were emailed on a daily basis until day 14 and then again on day 30.
Sample size was based on current volume of positive test results in June 2020 in Lancaster County, Pennsylvania. Before the start of the study, we conducted a power analysis suggesting that, if our endpoint was hospitalization and we hoped to detect a difference of 6.5% between either of the treatments arms and the placebo arm, we would have needed to recruit 151 patients per arm or a total of 453 patients for an appropriately powered study.
We did not perform an interim analysis and stopped our study due to resource limitations—including inadequate funding and a decreasing number of patients being diagnosed with COVID-19 infection in our area—in June 2021.
After enrollment, participants were randomized to 1 of the 3 groups, to receive a matched encapsulation of either vitamin C 1000 mg, melatonin 10 mg, or placebo (cornstarch) at bedtime for 14 days. Doses were chosen because they are easily obtained over-the-counter, and represented relatively little risk to most patients. Once-daily dosing allowed each patient in the trial to take an identical looking capsule in an identical way. Randomization was performed using random number seed to generate randomization sequence, blocked at 30 for every 30 subjects enrolled and randomized, ensuring there were 10 randomized to each of the 3 groups. Randomization was uploaded into REDCap and only visible to the data architect and the pharmacist who sent patients their assigned medication. Participants were blinded to their assigned group and were not paid for their time. All physicians and analysts were blinded to the participants' group until after data analysis.
Study supplement was mailed on the day of enrollment. On average, participants began taking their study drug by day 3 of the study. Participants completed an initial survey and then daily questionnaires for 14 days—the initial included background questions—that contained a survey of both symptom severity and quality-of-life impact. (See Figures 2 and 3) We determined the time to receive study medication by including a question on the daily survey regarding whether the patient had taken the study supplement in the past 24 hours. Thus, we were able to monitor which day of the survey they started taking the study supplement.
CONSORT flow diagram for supplements for COVID-19 study.
The rating scales regarding symptom severity and quality-of-life impact were modified from the WURSS and specific to infection with COVID-19 as determined during early pandemic phase evaluation and documentation at CDC symptom tracker.20,21 Although no longer validated if modified, the WURSS has been extensively used and, starting with some of the early validation/reliability studies, has been shown to be valid and reliable. Thus, in May 2020, we added questions about COVID-19 specific symptoms, including fever; chills; muscle/body aches; diarrhea; abdominal pain; loss of taste or smell; headache; and lack of energy. [See Figures 2 and 3]
The symptom section asked patients to rank the following symptoms on an 8-point scale from 0 (do not have this symptom) to 7 (greatest symptom severity): fever, chills, cough, shortness of breath, sore throat, muscle/body aches, diarrhea, abdominal pain, nausea, vomiting, loss of smell or taste, headache, and lack of energy. The quality-of-life impact section asked patients to rank on an 8-point scale the effect these symptoms had on their quality-of-life in the following areas from 0 (no impact at all) to 7 (severe impact): think clearly, sleep well, live your personal life, breathe easily, work inside the home, work outdoors around the home, accomplish daily activities, interact with others, walk, climb stairs, and exercise. Low scores indicated that symptoms were minimal or quality of life was not affected.
Assessment of our data began in July 2021 by team statisticians at the PMLGH Research Institute using an intention-to-treat analysis, analyzing survey data regardless of whether participants took their study drug. The primary endpoint of this study was to determine whether treatment with Vitamin C or melatonin resulted in a change in symptom score trajectory over the course of the study compared with those taking placebo. A secondary endpoint was to determine the course of symptom progression in outpatients with COVID-19. (See Table 2–5)
For statistical analysis, the symptom and quality-of-life scores were totaled per participant and then regression was calculated as a daily difference compared with baseline. Thus, on entry to the study, a participant's total score was used as baseline and then each day the difference from that initial score was calculated. If scores remain unchanged, then from one day to the next the graphical representation would be horizontal, whereas a great change from one day to the next would be represented as nearing vertical. (See Figures 2 and 3)
Supplements for COVID-19 symptom score regression analysis.
Supplements for COVID-19 quality of life regression analysis.
Initial survey was filled out electronically by participant after establishing REDCap interface and completed with the research coordinator's telephone-based assistance to clarify any concerns.
Daily survey (2 Pages) was sent electronically by REDCap to participant daily from day 2 until day 14 and once again on day 30.
Symptoms and quality-of-life impact scores were examined separately, using the same method so that the daily difference in total score for each was analyzed using a spline regression analysis. Spline regression analysis allowed us to break the study period into sections based on the inflection points in the change in scores. This involved dividing the 14 day periods into 3 segments that best fit the curves of the graphs (days 1 to 3, days 3 to 9, and days 9 to 14). We then compared the slope of each line segment to determine if there was a difference in the rate of recovery for each group and compared this to placebo. This comparison described the differences in rate of symptom resolution or quality of life resolution compared with placebo. A lack of improvement would thus mean a difference of zero, and on the graph would be represented as zero slope or a horizontal line. (See Figures 4 and 5)
We conducted reliability estimates with a standardized Cronbach's α for baseline scores, all daily responses and for each day. These were calculated for the combined 20 items and for the symptom and activities subsections (See Table 6). We found satisfactory reliability estimates for our modified WURSS for assessment of symptoms of COVID 19 patients.
Changes to Study Protocol
We initially proposed recruiting only patients age 50 and older. Due to concerns about low enrollment, criteria were expanded in October 2020 to enroll patients age 40 years and older. In January 2021 we asked participants to fill out an additional daily survey at the 30-day mark, identical to those completed on days 2 through fourteen, to determine if they were still experiencing symptoms. Fifty patients completed the survey, and the results are included in Table 2 and 3 but are not part of the regression analysis. Notably, the predeclared primary and secondary endpoints did not change during the course of the research or during post hoc analysis.
Baseline Demographic Characteristics by Group of All Study Participants
Average Symptom Score for Participants per Group on Representative Days
Average Quality-of-Life Score for Participants per Group on Representative Days
Results
A total of 104 patients were recruited; 6 dropped out. Of the 6 who withdrew from the study, 2 did not receive their medication; 1 reported drowsiness after 1 day; 3 dropped out without giving a reason. The remaining participants (n = 98) randomized to the 3 arms (34 received placebo, 32 received vitamin C and 32 received melatonin) had similar distribution of age, comorbidities (including diagnosis of cancer, diabetes, COPD, asthma, and high blood pressure), rates of vaccination and rates of receipt of monoclonal antibody infusion therapy, which became available in Lancaster in February 2021. Study analysis was by original assigned group, indicating an intention-to-treat. There were no deaths, hospitalizations or significant adverse events among participants in this study. See Table 1.
There were no significant differences between the 3 groups when comparing baseline survey scores for symptoms or quality of life (activity). (see Table 1) There was no significant difference in first or third segments of the spline regression—correlating to survey scores from days 1 to 3 and days 9 to 14—in comparison of the change in total symptom survey and quality of life scores. (See Tables 4 and 5; Figures 4 and 5) There was also no difference in second segment symptom score slope between those participants taking placebo (coefficient: -1.09; 95% confidence interval (CI): -1.39 to -0.8) and vitamin C (coefficient: 0.22; 95% CI: -0.2 to -0.63; P = .31).
Symptom Score Spline Regression Analysis Slope Coefficients
Quality-of-Life Score Spline Regression Analysis Slope Coefficients
Tests for Reliability Estimates with a Standardized Cronbach's Alpha for Baseline Scores, All Daily Responses and for Each Day
The group taking melatonin, however, had a different second segment slope (coefficient: -0.63; 95% CI: -1.02 to -0.21; P = .003) compared with the placebo group, demonstrating that during the second time period, days 3 to 9, patients in the melatonin group began to feel better faster.
Similarly, the quality-of-life impact analysis demonstrated that compared with the group taking placebo (coefficient: -0.71; 95% CI: -1.11 to -0.3) the group taking vitamin C (coefficient: 0.45; 95% CI: -1.03 to 0.12; P = .12) improved at the same rate. However, the group taking melatonin had a different second segment slope (coefficient: -1.16; 95% CI: -1.75 to - 0.57; P < .001) compared with the placebo group, demonstrating that during the peak of their illness, days 3 to 9, patients in the melatonin group had faster improvement in quality of life. The changes identified here were between survey scores on consecutive days of the study, and thus represent small changes over a brief period of time.
By study day 14, all 3 groups were approaching a plateau in improvement in both symptoms and quality of life. (See Figures 4 and 5) The results of the final survey demonstrate that at 30 days, many participants were still experiencing some symptoms. Many participants' quality-of-life were still impacted as well; the 30-day results were similar to results noted at day 14. [See Tables 2 and 3]. Analysis of raw data for each of the symptoms and quality of life indices did not reveal any other notable differences between the 3 groups.
Notably, we also asked about duration of sleep on each daily survey. Comparing the patient reported sleep duration among the groups, we could not detect any difference between the duration reported by patients in the melatonin arm from beginning to end of study nor between those using melatonin and those in the vitamin C or placebo arms.
Discussion
In this study, analysis of survey results allowed us to track daily symptom and quality of life improvement. Change from baseline symptom score and baseline quality-of-life score indicated improvement, with a steeper spline regression slope indicating faster improvement. Participants taking vitamin C 1000 mg once daily had similar regression patterns to those on placebo, indicating no difference in improvement rates. On the other hand, participants taking melatonin 10 mg once daily had faster improvement of symptoms and quality-of-life scores.
This trial was designed to look for a difference in the symptom and quality of life course of COVID 19 patients taking melatonin 10 mg or vitamin C 1000 mg once daily versus placebo and to model disease trajectory. While vitamin C 1000 mg once daily appeared to offer no benefit, we did detect a statistical difference in the course of those taking melatonin 10 mg once daily. It remains unclear, however, whether this statistical difference represents a clinically significant benefit. Larger, more robust trials should be conducted to investigate whether melatonin supplementation can indeed alter the course of COVID-19 infection in a clinically meaningful way. Patients infected with COVID-19 whose recovery process could be accelerated by safe, accessible supplements might return to work and life activities faster and thus meaningful interventions may have an impact on society as a whole.
Limitations of this study include the small number of enrollees (n = 104), which is too small in most cases to generate adequate power. Further, there were delays to participants taking their study drug, because of time lag from date-of-symptom onset to testing for COVID-19 infection; from testing to knowing results; from result to enrollment; and from enrollment to delivery of study drug. When testing capacity improved over the course of the study period, delays improved as well. While the majority of participants were taking their study drug by the third day after enrollment, that may have been up to 7 days into illness course. A delay from diagnosis to treatment has been shown to minimize any positive effect of treatment.8,16,17 Nevertheless, the concept of this study is generalizable because many patients do not present for evaluation on the first day of symptoms and there may be a lag time while accessing and awaiting laboratory test results.
The strengths of this study include accessibility of our surveys, in English and Spanish, and to those isolated in their homes. There was a very low drop-out rate (6%) and ultimately 76% (75/98) of remaining participants completed at least 10 surveys. This study has a robust design of randomized, double-blind, placebo-matched control trial.
Conclusion
We describe the use of a modified WURSS scale to measure outcomes of treatment targeting patients with COVID-19 infection. This analysis suggests the need for further validation and reliability study as a modified version would be useful in future studies in patients with mild-to-moderate viral-COVID-19 infections.
Vitamin C has long been touted as a potential remedy for various viral illnesses, yet a recent trial of vitamin-C (8000 mg) and zinc gluconate supplementation, either alone or in combination in outpatients with COVID-19 infection in Ohio and Florida failed to show a difference in mean time to achieve a 50% reduction in symptoms.22 Subjects in that study were reportedly enrolled within 1 to 2 days of testing positive although the duration of symptoms before enrollment was not analyzed; there was no placebo control; and there was no quality-of-life impact analysis.22 In another study, inpatient analysis of high dose intravenous vitamin C (6g/24 hours) added to standard of lopinavir/ritonavir and hydroxychloroquine in hospitalized COVID-19 patients demonstrated no benefit with regard to outcomes of length of hospital stay, length of ICU stay, oxygen demand or mortality compared with standard care alone.23 Finally a meta-analysis of 6 studies (n = 572 patients) revealed that Vitamin C, when given orally or intravenously, does not improve mortality, ICU length of stay, hospital length of stay, or need for mechanical ventilation.24
Our study is the first of which we are aware to describe testing melatonin in ambulatory patients with COVID-19 infection, although retrospective analyses have suggested that patients who take melatonin supplement are at decreased risk for manifesting symptoms of COVID-19 and have decreased mortality risk if they do contract the infection.25,26 Although we had hypothesized that any effect melatonin might have on potential outcomes would be secondary to the amount of sleep those using melatonin experienced, post hoc analysis revealed there was no difference between the average reported sleep duration of those in the melatonin arm compared with sleep reported by those in the other two arms. Thus, melatonin's impact on disease course may be related to its immunomodulatory and antioxidant effects.13 We suggest further larger scale investigation to determine reproducibility and elucidate whether melatonin can truly hasten recovery and return to activity, as there is a clinically significant role for treatments for those with mild-to- moderate COVID-19 infection. Further, it would be valuable to determine whether melatonin may have any impact on so called “long haul syndrome.”
It is now apparent that the pandemic and infection with COVID-19 will challenge us for the foreseeable future. Trials conducted with a WURSS or modified WURSS scale—including studies of low risk, accessible options—may prove valuable as we try to help patients overcome their illnesses and return to routine household, work, and school activities. Over-the-counter supplements such as vitamin C and melatonin are relatively safe, accessible and affordable; knowledge about their utility will aid primary care providers as they advise patients. Primary care providers will continue to need treatment options for first time and break-through infections; thus, we urge continued study to clarify the role these supplements may play, if any, in the care of patients with COVID-19.
Acknowledgments
Jessica Mace, PharmD with Penn/Lancaster General Health in Lancaster PA; Pharmacist assisted with study, provided essential materials was not paid. Kelly Fogleman, MPT with Penn/Lancaster General Health in Lancaster PA, Volunteer assisted with study, conducted research (hands-on conduct of the experiments and data collection) was not paid, Peter Rippberger, DO with Penn/Lancaster General Health in Lancaster PA, Volunteer assisted with study, conducted research (hands-on conduct of the experiments and data collection) was not paid., Michael Horst, PhD with Penn/Lancaster General Health in Lancaster PA Consultant and advisor, was not paid, Brian Young, MD with Penn/Lancaster General Health in Lancaster PA Consultant and advisor, was not paid.
Notes
To see this article online, please go to: http://jabfm.org/content/33/5/695.full.
This article was externally peer reviewed.
Conflict of interest: The authors report no conflicting or competing interests.
Funding: This study was funded by the Lancaster General Foundation Mary Elizabeth Roth Fund. The fund and its board had no role in the study. Funds paid only for study drugs purchased through usual pharmacy outlets and shipping costs to deliver study drug to participants. All authors and consultants associated with this project volunteered their time.
Clinicaltrials.gov registration number: NCT04530539
- Received for publication December 18, 2021.
- Revision received March 4, 2022.
- Revision received March 21, 2022.
- Accepted for publication March 25, 2022.