Abstract
Introduction: This review aims to determine whether active adults who begin statins and develop myalgia reduce or stop activity to become less symptomatic. If this occurs, strategies to mitigate symptoms are explored. Should these strategies fail, the question of whether exercise is an adequate alternative to statin therapy is addressed.
Methods: PubMed, Google Scholar, and the Cochrane Database were searched with keywords designed to retrieve information on statin myopathy in exercising adults.
Results: Statins are well tolerated by most people who exercise; however, caution is warranted in those who exercise at high levels, in the elderly, and in those receiving high-dose therapy. Several strategies improve statin tolerance while maintaining exercise levels, based on low-quality evidence. If statins are not tolerated, a continuing physical activity program can provide equivalent or superior cardiometabolic protection.
Conclusions: Statins may occasionally present a barrier to physical activity. A number of strategies exist that can reduce the risk of myopathy. If a choice between exercise and statins becomes necessary, exercise provides equal benefit in terms of cardiovascular protection and superior mortality reduction, with improved quality of life.
- Aged
- Exercise
- Hydroxymethylglutaryl-CoA Reductase Inhibitors
- Muscles
- Muscular Diseases
- Myalgia
- PubMed
- Quality of Life
Wider indications, lower treatment thresholds, and higher dosing ranges for statin therapy in the primary prevention of cardiovascular disease (CVD) have been common trends in North American guidelines for lipid management since 2012.1⇓–3 Adherence to the American College of Cardiology and American Heart Association guidelines would result in treatment recommendations based on age alone: all white men aged 63 to 75 years and all white women aged 71 to 75 years, with otherwise optimal risk factors.4 Several meta-analyses have suggested event reduction beyond age 75.5⇓–7 Relative event reduction is thought to be at least as large in patients at low risk for CVD as it is for those at high risk, admittedly with much larger numbers needed to treat. Although individuals without known CVD are at lower absolute risk, nearly half of vascular events may occur in this population.5
The demonstrated effectiveness of statins in primary prevention, together with the increased availability of generic high-potency formulations, makes preventive therapy attractive for large numbers of people on a population basis. In return for a cardiovascular benefit to 1 person in a low-risk population, many people will be treated who could not possibly benefit.8 For this reason, the adverse effects of a statin intervention must be kept extremely low, as potential harm remains the same for the numerous people who receive no treatment advantage.2,9,10 Unfortunately, the adverse effects of statins have not been studied and reported as systematically as the benefits.11
Although the vast majority of the North American population is sedentary,12,13 significant numbers undertake physical activity (PA) on a regular basis. There is increasing participation in endurance14 and ultraendurance sports,15 with ongoing increased participation by women.14 Runners ≥40 years old now constitute almost half of marathon participants in the United States.14 Others simply remain active as a health and lifestyle choice. Among the known triggers for statin myopathy, exercise is most frequently associated with morbidity. 16⇓–18 Because PA is a potent factor in reducing both cardiovascular events19 and mortality,20 it is important that we not interfere with the implementation of an effective lifestyle option by introducing a drug intervention that may impair the ability to exercise. At the same time, there is clear observational evidence for the benefits of statin therapy, even at high levels of exercise,21 so a combination of lifestyle and drug interventions is optimal if this conforms to patient preference.
The objectives of this review are 3-fold:
To determine whether statin administration increases the risk of muscle symptoms and leads to reductions in PA among exercising people without known CVD
To determine what might be done to mitigate any increase in symptoms to allow the increased protection against CVD provided by statins
If there are intolerable symptoms despite attempts at mitigation, to determine whether exercise alone can provide protection against CVD and mortality protection comparable to the known and proven benefits of statin therapy.
Methods
A narrative review was undertaken to determine the influence of statin therapy on muscle symptoms and activity levels among exercising adults in primary prevention. Further information was accumulated to gather data on symptom mitigation and therapeutic alternatives as stated in the objectives listed above.
Search Strategy
PubMed was originally searched for articles available in English using the search keywords statin* AND (muscle OR myopathy) AND (exercise OR “physical activity” OR fitness) NOT (rehabilitation OR cancer). The exclusion terms were to limit the retrieval of cardiac rehabilitation studies that address secondary prevention and cancer, which are outside the scope of this inquiry. Bibliographies of relevant articles were searched for additional references. The same search terms were used in similar queries of the Cochrane Database and Google Scholar. Similar PubMed searches were made for meta-analyses relating to health outcomes for either statin therapy or the grouping of exercise keywords. Outcomes were entered as mortality OR “cardiovascular disease” OR “cardiovascular events.” These articles were used to evaluate, where possible, the relative effects of exercise and statin therapy on mortality or cardiovascular outcomes.
Study Selection
The Strength of Recommendations Taxonomy22 was used to evaluate recommendations and study quality (Table 1). In comparing individual benefits of statins and exercise, level 1 evidence was selected for trials related to statins, as numerous randomized controlled trials (RCTs), systematic reviews, and meta-analyses were available. The most reliable evidence for exercise outcomes was from prospective cohort studies because of constraints imposed by problems with blinding, allocation concealment, and adherence. Exercise studies therefore included level 2 and 3 evidence. Articles on secondary prevention, animal studies, and studies of populations with cancer or chronic disease were excluded.
Strength of Recommendation Taxonomy
Results
Pathophysiology
Statin effects on muscle are the subject of some controversy. Terminology varies among clinical advisories,23⇓–25 and the American Heart Association/American College of Cardiology classification is used in this discussion, where myositis implies inflammation marked by an increase in creatine kinase (CK) and myopathy refers to any muscle event (Table 2).
Definitions of Muscle Syndromes
Unaccustomed exercise induces muscle injury, with myalgia and CK concentrations peaking 2 to 3 days after the activity. There is evidence of increased inflammatory response following exercise, with the release of proteolytic enzymes and reactive oxygen species in preparation for tissue regeneration. This cycle of damage and repair is considerably blunted by repeated bouts of similar exercise.26
The mechanism for muscle toxicity associated with statin therapy is not well understood. A blinded crossover study27 established that symptoms of pain and weakness can reliably be related to statin administration without an increase in CK. Despite this, there is clear evidence from a blinded biopsy study of slight but consistent damage to myocyte structural integrity in asymptomatic subjects taking statins.28 CK elevation is more common among exercising people who are taking statins,29 but an increase in the enzyme is not a consistent feature of symptomatic statin-induced myopathy.
Ubiquinone, or coenzyme Q10, is produced via the cholesterol metabolic pathway, and concentrations are reduced by statin administration.30 It is involved in electron transport in mitochondria, and deficiency could impair mitochondrial energy metabolism in muscle,31 although evidence of this is conflicting.32 Interference in this pathway can also destabilize muscle membranes during activity.33
Impact of Statin Myopathy in Exercising Adults
Statin-related adverse effects are reported to be as low as 1% to 5%34,35 in the large RCTs examining drug effects on CVD events and mortality. Some of these trials have excluded up to 30% of participants in the prerandomization run-in phase, some of whom may have had muscular symptoms.16 The quality of reporting of myalgia in these trials also is highly variable.36 There has not been much difference in muscle symptomatology reported between subjects and controls in large RCTs.37,38 There may be a tendency to attribute background muscular symptoms to the medication, be it active statin or placebo. This so-called nocebo effect39 attributes harm to the inactive intervention. With such a large component of similar symptoms present among the controls, it is challenging to demonstrate adverse effects of the statin because the signal can be overcome by the noise, so to speak.
Pain incidence seems to be higher in observational studies. More recent studies specifically targeting statin myalgia at a high dose are the Prediction du Risque Musculaire en Observationnel (PRIMO)18 and The Effect of Statins on Skeletal Muscle Function and Performance (STOMP)40 studies. The former is a longitudinal observational examination of 8000 hyperlipidemic patients taking statins, 10.5% of whom reported myalgia (number needed to harm, 26). The STOMP study was an RCT showing a 9.4% incidence of reproducible myalgia with statin treatment, approximately double the frequency in the control group. A meta-analysis of high-quality prospective, observational studies41 found an odds ratio of 2.3 for muscle symptoms with a statin over placebo. A review of registry data and observational studies reports myopathy ranging from 7% to 29%.42
True statin-related symptoms are usually generalized and symmetrical, characterized by aching, pain, tenderness, or cramping. There may be muscular weakness.42 Regardless of the type of study, the incidence of severe myopathy marked by increased CK—exceeding 10 times the normal value—or of rhabdomyolisis is low (probably <0.1%).18
Milder muscular symptoms may be sufficient to give the patient a compelling reason either to stop the medication or to become less physically active. A number of studies have examined the relationship of statin medication to a change in PA status. Sinzinger and O'Grady,43 in a case series of 22 young elite athletes with familial hypercholesterolemia, found that 80% of them were unable to tolerate a statin at any dose while maintaining their incident activity level. Bruckert et al,18 in an observational cross-sectional study of 8000 unselected patients taking high-dose statins, noted muscular symptoms in 10.5% of subjects, 38% of whom were unable to tolerate a moderate level of PA. Lee et al,44 in a 7.2-year prospective cohort study of 8000 men over 65 years of age, found that subjects taking statins had a 10% decline in PA and displayed more sedentary behavior compared with controls. New statin users showed the most rapid decline in activity. An RCT examining 420 healthy, statin-naive adults showed a significant increase in CK and myalgia after 6 months of statin administration.40 PA status in younger subjects was well preserved, but the group over 55 years old showed a significant decline compared with controls. On the other hand, Williams and Thompson,45 in a prospective cohort study involving 78,000 statin-naive runners and walkers followed for 7.2 years, found a reduction in activity among all subjects with hypercholesterolemia, whether taking statins or not. It was postulated that this may represent reverse causality, suggesting that inactivity might lead to high lipid values, which in turn lead to an indication for statin treatment. Panza et al,46 in an RCT involving 418 statin-naive adults showed a reduction in PA over 6 months that was equal in those randomized to statins and in controls. This was true at all activity levels, but there were very few subjects in this trial with high levels of activity. These and other studies relating statin administration to PA levels are summarized in Table 3.
Studies Relating Statin Use and Physical Activity
These studies leave room for optimism. While the majority of subjects given medication may reduce PA levels over time, this does not seem to be an outcome specific to statins. Caution with statin administration might, however, be appropriate in those engaging in high levels of exercise, those taking high-dose statins, and the elderly. Any muscular complaint following statin administration should be evaluated, and an attempt to mitigate symptoms should be considered given the clear benefits of medication demonstrable at all levels of PA.
Mitigation of Statin Myopathy
As indications for statins become more liberal, those who regularly exercise become candidates for therapy simply based on age and accumulation of modest risk.54 CK concentrations in these people are frequently elevated. While CK concentrations can indicate muscle damage, they are not reliable as an indicator of myopathy.27 Values up to 10 times the upper limit of normal can be present without symptoms and do not warrant statin discontinuation.55 It is recommended that CK concentrations exceeding this should, in the absence of an alternative explanation, trigger discontinuation.56 CK concentrations tend to be chronically elevated in those who habitually exercise and have been reported to be as high as 90 times the upper limit of normal in ultramarathoners.55 For this reason it is probably wise to obtain a baseline CK value before starting statin therapy in those with a high level of activity to properly assign a source for the enzyme elevation.16
Since the addition of statins can contribute significant further reduction in cardiovascular risk in exercising adults, a number of suggested strategies facilitate the use of medication if it is the preferred treatment plan:
Ensure that a stable and continuing exercise program is established before introducing statins. Myopathy is most common upon first starting an activity program.57 Regular prior exercise protects against statin myopathy.58,59
Reduce statin potency. A large case series suggests this may be effective.53 Some suggest the use of a low dose of a more potent statin.36 The only existing RCT comparing myalgia at high and low doses of statins actually showed no difference.60 Since there is no clinical trial–based evidence for an additional benefit of high-dose statins against mortality in primary prevention,61 and since no study has established valid low-density lipoprotein targets,1 it would seem reasonable to be content with a low dose if a statin is to be used.
Reduce dosing frequency. Using a longer-duration preparation such as rosuvastatin 5 to 10 mg once weekly62 or twice a week63 has been effective in reducing myalgia. CVD outcomes from the use of this strategy after 8 years do not show significant survival benefit thus far.64
Change the statin preparation. A number of studies suggest that fluvastatin is least likely to cause myopathic symptoms.18,35 This may be because of its low lipophilicity, high hepatic first-pass metabolism, and high protein binding, along with the availability of a slow-release preparation.35 It has the lowest incidence of rhabdomyolysis.34 A blinded randomized trial of fluvastatin XL, ezetimibe, and a combination of the 2 showed the lowest myalgia incidence to occur with the combination.65 There was, unfortunately, no placebo control. There is evidence for improved cardiovascular outcomes with a statin/ezetimibe combination.66
Vitamin D deficiency. Vitamin D deficiency can produce myalgia similar to statin myopathy.67 Observational evidence from a meta-analysis has associated the 2.68 The best prospective cohort study suggests that vitamin D repletion can improve symptoms in 88% of statin-intolerant patients at 6 months.69 No RCTs are available. This is a harmless intervention but requires expensive testing and follow-up, and it is based on conflicting and low-quality evidence.
Coenzyme Q10 replacement. Statin therapy leads to low coenzyme Q10 levels. Unfortunately, a recent meta-analysis of 6 RCTs suggests no advantage to coenzyme Q10 replacement over placebo.70 Available supplements are not harmful, but can be expensive.
Alternative drugs. Niacin and fibrates have been shown to be effective when used alone to prevent CVD,71,72 but they have not been exclusively studied in primary prevention. Both have been associated with myopathy.25,73 Ezetimibe has no myopathic effect, based on a systematic review.74 In combination with colesevelam it has reduced myopathy in statin-intolerant patients.36 Ezetimibe alone currently has no evidence for improved cardiovascular outcomes.75
New drugs. PCSK9 inhibitors have recently become available, and phase II trials have suggested low rates of myalgia. The highest quality published study of alirocumab showed a myalgia rate of 5.4% versus 2.9% for placebo.76 Event rates for this finding were low, but the difference was significant. It is probably too early to present this drug class as a alternative to statins for this indication.
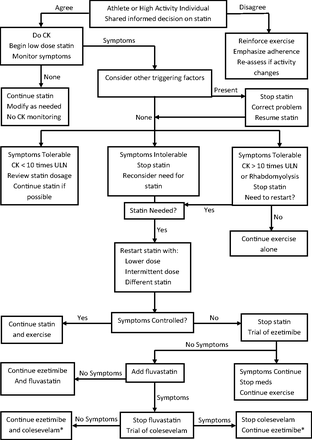
A summary algorithm in presented in Figure 1. There is a reasonable chance for success in reversing the considerable nocebo response to statin administration; however, if manipulating medications interferes with either the ability or motivation to continue PA, alternatives should be discussed with the patient. Physical inactivity should not become a prerequisite for successful statin treatment. Exercise is among the most powerful interventions in the primary prevention of CVD.78
Management of statin myopathy in exercising adults. *Lowers low-density lipoprotein (no evidence of cardiovascular disease event reduction109). CK, creatine kinase; ULN, upper limit of normal.
Comparing the Benefits of Drug or Exercise Interventions Used Alone
The primary prevention population without known CVD comprises a significant portion of habitual exercisers and athletes. Where a statin indication exists, there is established benefit of a combination of exercise and medication against mortality, even at high levels of activity21,79. If the addition of a statin is followed by increased muscle symptoms and attempts at mitigation fail, there may need to be a choice between modalities. A comparison of the benefits of statins and exercise in primary prevention is therefore appropriate.
Of the many large placebo-controlled statin trials, only 4 examined the primary prevention population as the sole focus.80⇓⇓–83 The meta-analyses of statins in primary prevention are summarized in Table 4. Some of these analyses contained trials populated with up to 20% of patients in secondary prevention. The presence of some subjects with established disease may have made estimates of statin benefit overly optimistic.
Meta-Analyses: Statin Treatment Effects in Primary Prevention
Generally, statins reduce CVD events more than all-cause mortality in primary prevention. Cardiovascular events are reduced by 20% to 40%,84,86,89⇓–91 with a number needed to treat between 5690 and 7791 for men and women. Benefit against all-cause mortality cannot always be demonstrated because of the small numbers of events in this population. In studies showing a benefit,90,91 the number needed to treat is much higher: between 9690 and 167.91 A benefit against mortality for women alone has been shown,89 but this is not a uniform finding.86,88 There is limited evidence that statins can reduce CVD events in elderly patients,6 but there is no benefit against mortality, and overall treatment in this age group remains controversial. Several meta-analyses provide evidence that high-dose statin therapy, as opposed to moderate doses, provides additional protection against cardiovascular events, but not all-cause mortality, in secondary prevention,85,92,93 but no such benefit has been found in primary prevention.61
The benefits of exercise extend equally to reductions in CVD and mortality, with a consistent dose response (Table 5). Variable but predictable risk reduction occured in primary care meta-analyses, with estimates ranging from 11% to 57%. The greatest benefit is reported in subjects evaluated by fitness testing or accelerometry rather than by self-report.21,95 The tendency to overestimate PA leads to the underestimation of the exercise effect. This trend is well illustrated in the 2009 Canadian Physical Activity Survey, where 52.5% of adults reported undertaking moderate exercise. Accelerometry data showed that the true moderate activity level in this population was only 15%.98
Exercise Benefit in Primary Prevention: Meta-analyses of Prospective Cohort Studies
Studies of exercise in primary prevention use prospective cohorts and cannot prove causation, but the findings are remarkably consistent, and the magnitude of benefit seems to be equivalent to that of statins. Moderate activity can give the same protection against mortality as statins when applied to a sedentary population.21 Vigorous activity can produce a relative risk reduction as high as 60%.21 Meta-analyses that compare exercise with statins for secondary prevention show a comparable effect.99
PA may actually be superior to drug therapy in some respects:
In addition to comparable CVD event reduction, there is a consistent benefit against mortality in primary prevention, which is less apparent for statins.
Exercise benefit may be better for women than men.94,95 The benefits of statins for women has been more difficult to demonstrate.
There is benefit in the elderly100; this is still controversial for statins.
There is long-term evidence of the benefits of exercise.101 There are no long-term studies of statins,102,103 but follow-up beyond 10 years in a few large trials does show continued benefit.104
Exercise can delay or prevent diabetes onset.105 Statins modestly increase incidence.106
Higher exercise levels are associated with reduced levels of obesity.107 Statins have no influence on body mass index.
Exercise is associated with improved quality of life,108 which is not demonstrated with statin therapy.
While a combination of PA and drug therapy may be preferable in high-risk patients,21,46 exercise can provide a satisfactory alternative if patients are unable or unwilling to adhere to statin therapy.
Discussion
The highest-quality evidence found in this review suggests that, despite being at higher risk for myalgia, the majority of exercising adults are likely to see little impact of statin therapy on exercise intensity or duration.46 It is possible, however, that the significant nocebo effect—an adverse effect from the actual administration of a pill—has some detrimental effect on these parameters. There is reason for concern, based on lower levels evidence, that statins may adversely affect PA levels in high-performance athletes and in the elderly. In addition, high-dose statins are more likely to influence activity levels, but they afford no additional benefit over lower doses in preventing events in primary care.
This review found 1 publication examining the primary prevention literature regarding the impact of statin therapy on exercising adults.109 This was an editorial referencing an observational study,45 which related activity reduction to cholesterol levels rather than to statin administration. Reverse causality was suggested: inactivity led to high cholesterol, which triggered a statin prescription. No studies were found directly comparing PA and drug therapy interventions in the reduction of CVD events and mortality in this population. The finding of the relative equivalence of the 2 interventions is in line with meta-analyses done in secondary prevention by Naci and Ioannidis,99 which found them to be statistically equivalent.
Limitations
This investigation follows 3 separate lines of enquiry and attempts to draw them together to form conclusions that may be useful to generalists. It is a traditional review rather than a systematic review. Selection bias may be a risk.
The review shares the shortcomings of the existing evidence. Much of the exercise literature is observational and self-reported. Much of the statin myalgia data are poorly reported, even in the many otherwise well-done RCTs. When observational studies are the best evidence available, they can suggest only association, not causality.
Future Directions
There is a need for a large RCT of statin tolerance in both statin-naive high-performance athletes and in the elderly population, looking at drug adherence and exercise performance outcomes. Such a trial will probably be difficult to design for the inclusion of athletes, because these subjects are likely to be intolerant of any intervention that might detract from exercise performance. The statin intervention could be low dose, since there is little evidence for any benefits of high-dose therapy in primary prevention.
Conclusions
Conclusions and levels of evidence are outlined in Table 6. Statin-induced myopathy is more prominent among exercising adults, but many people taking statins are able to maintain their desired level of activity. People at higher risk for symptoms include high-performance athletes, the elderly, and those taking high-dose statins.
Conclusions Regarding Statin Use in Exercising Adults
Some strategies may increase statin tolerance based on low to moderate levels of evidence. Whether these strategies have a real benefit or whether they simply blunt the influence of the nocebo effect, it is usually possible to use these measures successfully to improve statin tolerability. If this is not possible, a robust and lifelong exercise habit is probably an adequate alternative to statin therapy in the primary prevention of cardiovascular events and mortality.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
- Received for publication March 1, 2016.
- Revision received June 7, 2016.
- Accepted for publication June 13, 2016.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.
- 48.
- 49.
- 50.
- 51.
- 52.
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.
- 97.
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵