Abstract
The incidence of obstructive sleep apnea (OSA) has reached epidemic proportions, and it is an often unrecognized cause of perioperative morbidity and mortality. Profound hypoxic injury from apnea during the postoperative period is often misdiagnosed as cardiac arrest due to other causes. Almost a quarter of patients entering a hospital for elective surgery have OSA, and >80% of these cases are undiagnosed at the time of surgery. The perioperative period puts patients at high risk of apneic episodes because of drug effects from sedatives, narcotics, and general anesthesia, as well as from the effects of postoperative rapid eye movement sleep changes and postoperative positioning in the hospital bed. For adults, preoperative screening using the STOP or STOP-Bang questionnaires can help to identify adult patients at increased risk of OSA. In the pediatric setting, a question about snoring should be part of every preoperative examination. For patients with known OSA, continuous positive airway pressure should be continued postoperatively. Continuous pulse oximetry monitoring with an alarm system can help to prevent apneic catastrophes caused by OSA in the postoperative period.
- Obstructive Sleep Apnea
- Opioids
- Pediatrics
- Respiratory Failure
- Respiratory Tract Diseases
- Screening
- Sleep Disorders
- Snoring
- Surgery
The Institute of Medicine, in a 2006 publication titled Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem, noted the widespread underdiagnosis of sleep-related problems by clinicians, and called for increased educational efforts to improve awareness, diagnosis, and treatment.1 Obstructive sleep apnea (OSA) is a condition in which the upper airway becomes obstructed during sleep, causing hypoxia, hypercarbia, fragmented sleep, and a variety of medical complications including daytime drowsiness and an increased risk of hypertension, diabetes, and cardiovascular disease. OSA is highly associated with obesity and is becoming increasingly common as the obesity epidemic continues.2
The perioperative period is a time of particularly high risk for patients with OSA because of the adverse effects of anesthesia, narcotics, and sedatives on OSA. A number of studies have found that patients with OSA undergoing noncardiac surgery have a higher incidence of postoperative hypoxia, respiratory failure, cardiac events, and intensive care unit (ICU) transfers than those without OSA.3,4 Unfortunately, ≥80% of patients with OSA are unrecognized before surgery, putting them at increased risk of complications during the perioperative period.5
Family physicians believe OSA is important clinically but often do not address sleep issues. A study of 227 patients and 22 family physicians in 2 family medicine practices found that knowledge of OSA among family physicians was high, and 82% of physicians felt that OSA was very or extremely clinically important. However, physicians documented sleep complaints from patients' review of systems forms only 24% of the time, and only 11% of patients with sleep complaints received further workup. The authors noted that “Good physician knowledge and appropriate attitudes about OSA did not translate into high rates of documentation or clinical investigation.”6 There have been numerous articles on this topic in the surgical and anesthesiology literature, with recent guidelines from the American Society of Anesthesiologists (ASA) and the Society for Ambulatory Anesthesia addressing the issue of perioperative management of patients with OSA.7,8 However, a recent search of the family medicine literature revealed only 1 letter to the editor on perioperative aspects of OSA care.9
This article addresses the following questions: (1) Should patients contemplating surgery be screened for OSA? (2) Which screening tools are best suited for this purpose? (3) How should patients at risk of OSA or who have OSA be managed in the perioperative and postoperative state?
Methodology
A keyword search of the Cochrane Library, MEDLINE/PubMed database, and journal archives and current journals from January 1995 through July 2015 was performed using the terms obstructive sleep apnea or sleep apnea in the title or abstract, in combination with the terms screening, surgery, preoperative, postoperative, and perioperative. English-language, peer-reviewed studies assessing the effect of screening for OSA on perioperative morbidity and mortality were reviewed, as were general review articles that included recommendations for management of OSA during the perioperative period. The principal focus was perioperative management of adult OSA, but evidence was also sought for equivalent work on pediatric surgery and management.
Clinical Features
OSA (sometimes referred to as OSA syndrome [OSAS] when certain symptoms are present – see Table 1) is characterized by partial (hypopnea) or complete closure (apnea) of the upper airway despite ongoing respiratory efforts, leading to oxygen desaturation of the blood, and frequent arousals, resulting in restless sleep (technical terms are defined in Table 1). Adults with OSA typically snore loudly and often report waking from sleep with a choking sensation. The interference with normal sleep leads to daytime drowsiness and many complications in adults, including increased accidents and increased risk of hypertension, diabetes, obesity, stroke, myocardial infarction, and congestive heart failure10,11 (Table 2, column 1).
Pediatric OSA presents different symptoms than OSA in adults and is defined differently (Table 1 and Table 2, column 2). The most common cause of OSA in children is adenotonsillar hypertrophy, and adenotonsillectomy is the primary treatment for this condition, curing OSA in 70% of children.12,13 OSA is also associated with obesity and chronic upper airway inflammatory disorders, including sinusitis, allergic rhinitis, and asthma.14 Children are less likely to report daytime drowsiness. In children OSA often presents as learning difficulties, behavioral problems, and hyperactivity.15 In severe cases hypertension, cardiac dysfunction, and failure to thrive may be seen.16 Snoring is a common sign; as many as 40% of snoring children who are referred for evaluation by an otolaryngologist or sleep specialist prove to have OSA, and the absence of snoring makes a diagnosis of OSA much less likely.12,14,17
An illustrative case report exemplifies many of the features of OSA. A woman in her mid-50s was admitted to a hospital for workup of abdominal pain. Several days into her hospital stay she was given parenteral hydromorphone in the evening for pain. Five or 6 hours later she was found by the nurses to be cyanotic and apneic. She was successfully resuscitated and recovered fully after a 2-day ICU stay. She was evaluated by a cardiologist and neurologist. There was no sign of cardiac injury, and cardiac function was normal. She was felt to have had a drug reaction, possibly an interaction between the hydromorphone and a selective serotonin reuptake inhibitor she was taking. A subsequent workup for fatigue included a sleep study that showed mild sleep apnea, which was not treated.
Some months later she was admitted to the same hospital for an endoscopic study, and the evening before the procedure she was premedicated by the gastroenterologist with an intramuscular injection of meperidine. Some time later the nurses found her cyanotic and apneic, with an agonal heart rhythm. Resuscitation was unsuccessful. The family brought a lawsuit against the gastroenterologist and also against the family physician who had done the preoperative history and physical examination. After expert review determined that the patient had suffered a fatal apneic event caused by the effect of the meperidine injection on her OSA, the family physician was exonerated and dismissed from the case, which was subsequently settled out of court against the gastroenterologist for a substantial sum.
This case study exhibits several of the characteristics of OSA risk, from a near miss, through a failure to follow-up the OSA diagnosis with appropriate treatment or documentation following diagnosis, to a preventable death, and finally to complex litigation. This case parallels the findings of a recent review of medical-legal cases involving patients with OSA in the perioperative setting by Fouladpour et al, who found that “Slightly over half of the complications reported occurred in an unmonitored setting, and a substantial minority involved the use of opioids. These cases were most likely to be associated with death as the outcome.”21
Pathophysiology
Breathing is primarily controlled by central respiratory pacemakers in the medulla that interact with central and peripheral chemoreceptors. Central chemoreceptors in the medulla, pons, and cerebellum sense the pH of the central nervous system, which corresponds to the level (partial pressure) of carbon dioxide in the blood, whereas peripheral chemoreceptors, mainly in the carotid body, are primarily sensitive to changes in the oxygen concentration (partial pressure of arterial oxygen).22 The perioperative period is a particularly dangerous time for patients with OSA. General anesthetics, narcotics, and sedatives can worsen airway obstruction by enhancing upper airway muscle relaxation, reducing ventilation, and blunting arousal from sleep.5 Narcotics depress both the central response to hypercapnia and the peripheral response to hypoxia: at low doses they primarily decrease tidal volume; at higher doses they decrease the respiratory rate.22 Changes in rapid eye movement (REM) sleep during the postoperative period can also put a patient with OSA at risk. Pain and postanesthetic effects can reduce REM sleep, which is followed by a rebound increase in REM sleep 3 to 5 days after surgery. This REM rebound can worsen OSA because REM sleep is normally accompanied by unstable breathing, worsening of airway muscle tone, and a decrease in the arousal response to hypoxia, hypercapnia, and airway occlusion.24⇓–26 Adult and pediatric patients with OSA have been found to be highly sensitive to narcotics.27,28 One study of children found that those with OSA required approximately half as much morphine for pain relief as children without OSA, likely because of increased sensitivity to opioids caused by recurrent hypoxic episodes.27
Central sleep apnea is much less common than OSA among postoperative patients. A recent study of 376 postsurgical patients found very low preoperative rates of central sleep apnea, with a significant increase in central apneas on postoperative nights 1 and 3 after general anesthesia in both patients without OSA and those with OSA. However, the clinical significance of this was unclear.29
Epidemiology
The overall prevalence of OSA among the adult population is estimated to be as high as 1 in 4 men and 1 in 10 women, with a higher incidence among surgical patients.30,31 In a prospective, observational study, Finkel et al32 screened 2877 adult surgical patients with an OSA risk questionnaire. Of those patients, 661 (24%) screened as high-risk for OSA, and 81% of those did not have a diagnosis of OSA. Subsequent sleep studies showed the prevalence of OSA to be 82% among this high-risk group. The authors estimated the overall prevalence of OSA to be 22% among the adult surgical population.32 Another study found similar rates: of 1506 respondents, 26% (31% of men and 21% of women) met screening questionnaire criteria for high risk of OSA. The risk of OSA was even higher (57%) in obese individuals.31 The high rates of OSA among surgical patients are likely influenced by the higher prevalence of obesity in studies of these patients.5
The rates of diagnosed OSA among adults are lower. Using a nationally representative sample of the US adult population, Li et al estimated that the prevalence of diagnosed sleep apnea was 4.5% among all adults, 6.1% among men, and 3.1% among women. Age-adjusted prevalence was high among people with total obesity (men, 12.1%; women, 7.0%) or abdominal obesity (men, 10.9%; women, 4.6%).33
Among the pediatric population, OSA affects 1% to 6% of all children and up to 59% of obese children.14 Prevalence is higher among males than females, and higher rates have been found among African American children.34
Perioperative Risks of OSA
A number of studies of adult patients with OSA undergoing surgery found a significant increase in postoperative complications, ICU admissions, hospital length of stay, dementia, respiratory failure, and episodes of hypoxemia.3,24,35⇓–37 Although several large retrospective cohort studies indicated only mild risk38,39 or decreased risk of perioperative complications in patients with OSA,40 the controls in these studies were patients who were not actually proven to not have OSA, but were simply lacking OSA-related International Classification of Diseases, 9th Revision (ICD-9), diagnosis codes in their charts, thus confounding the result, considering that >80% of patients with OSA are undiagnosed.4,32,41 A meta-analysis by Kaw et al, comparing patients with OSA with controls who tested negative for OSA or screened at low risk of OSA (and excluding studies using “no ICD-9 codes for OSA” as controls), showed a uniform increase in perioperative morbidity from OSA, with significant heterogeneity of the effect among studies.3
Children with OSA are also prone to high rates of complications. One review of 111 cases of death or near-death following tonsillectomy in pediatric patients found that 57% of the children met criteria for being at risk for OSA. Among this group almost half of the events (46%) were related to apneas. In 2 of these cases the children were still in the postanesthesia care unit and their monitoring had been discontinued; thought to be asleep, they both died with one of their parents in their room.42
Data on mortality rates among adult inpatients are mixed. Although 1 study of patients undergoing arthroplasty revision showed an almost double rate of postoperative mortality among OSA patients compared to patients lacking a diagnosis of OSA,43 several other studies showed a lower mortality among OSA patients.38⇓–40 In a recent review and meta-analysis, Lyons and Mokhlesi commented extensively on conflicting mortality data.4 Regarding the paradoxical finding of decreased mortality among patients with OSA, they note that it may be related to the higher rate of obesity compared with patients without OSA and discuss the “obesity paradox” in which obesity seems to confer protection against mortality for a variety of conditions.44,45 They also note that imminent respiratory failure may be recognized and managed earlier in patients with OSA, and that patients without previously diagnosed OSA may nonetheless have OSA and its complications, leading to an underestimation of mortality rates. They conclude by noting that a number of studies have associated OSA with increased long-term mortality, and because a large number of patients with OSA remain undiagnosed, “the potential for benefit from recognition and referral during preoperative evaluation may be large.”
Preoperative Examination and Screening
During the preoperative history and physical examination, adults should be asked about a prior diagnosis of OSA, snoring, witnessed apneas, daytime drowsiness, and other symptoms associated with OSA (Table 2, column 1). Certain conditions predispose adult patients to a particularly high risk of OSA, including obesity, hypertension, diabetes, male sex, alcohol use, and large neck size (>43cm in males, >41cm in females). Chronic opioid use is also a risk factor for OSA, and chronic users have a higher prevalence of OSA (35% to 39%). Opioids cause peripheral chemoreceptors to become less sensitive to hypoxia; this occurs with acute opioid use and does not improve with chronic use.22
An ideal screening questionnaire for the primary care setting should be user-friendly, accurate, and generalizable to different target populations.50 Four tools validated for screening for OSA in surgical patients are the Berlin questionnaire, the ASA checklist, the STOP questionnaire, and the STOP-Bang questionnaire51 (Table 3). Although the STOP questionnaire is the easiest to use, the STOP-Bang modification improves the sensitivity and negative predictive value of the STOP questionnaire and has been shown to be accurate at predicting moderate to severe OSA in surgical patients (sensitivity for detecting OSA at different Apnea-Hypopnea Index [AHI]) cutoffs and severity levels: 84% for AHI ≥5; 93% for AHI ≥15, and 100% for AHI ≥30)24,50,52⇓⇓⇓⇓–57 (Table 4).
Pediatric questionnaires have been developed for the research environment, but a concise questionnaire that is practical in primary care has not yet been fully validated. A 2012 guideline from the American Academy of Pediatrics recommends that all children and adolescents be routinely screened for snoring at routine office visits, and should have a polysomnogram if in addition to snoring there are signs and symptoms of OSA58 (Table 2, column 2). A pediatric screening questionnaire called I'M SLEEPY (Table 5) was recently developed and validated in a population of 150 children.59 A score ≥3 is sensitive for a diagnosis of OSA. The questionnaire has a sensitivity of 82%, a specificity 50%, and a negative predictive value of 85% for screening for significant pediatric OSA. Although promising, the initial testing population was small, and further validation is needed to confirm its usefulness as a reliable tool. To date, no pediatric screening questionnaire has been validated with accuracy comparable to that of the STOP-Bang questionnaire for adults, and more research is needed in this area.
Pre- and Postoperative Management
Family physicians can be an essential part of early recognition of OSA, starting with screening during the preoperative history and physical examination. It is not sufficient to defer assessment solely to the surgical team: a study of 708 surgical patients with no prior diagnosis of OSA found that 38% had moderate and severe OSA based on preoperative polysomnography (AHI ≥15); the diagnosis of OSA in this group of patients was missed 60% of the time by anesthetists and 92% of the time by surgeons. This study also noted that use of the STOP-Bang questionnaire would have identified 93% of these patients as being at risk of OSA.41
We propose that all adult preoperative patients should, at a minimum, be screened using the STOP or STOP-Bang questionnaire. Primary care physicians screening a pediatric population should ask about snoring and can use the I'M SLEEPY questionnaire. A screening questionnaire can be made a routine part of standard preoperative examination forms both in paper charts and in electronic health records.
Primary care physicians who identify patients at high risk of OSA based on their history and physical examination and a screening questionnaire should consider referring those patients for a sleep medicine consultation, including a screening home sleep study, or complete polysomnography (time permitting), and appropriate OSA treatment.61 Alternatively, patients can be “flagged” as having a high probability for undiagnosed OSA and proceed to surgery if delaying the surgery is deemed unacceptable.
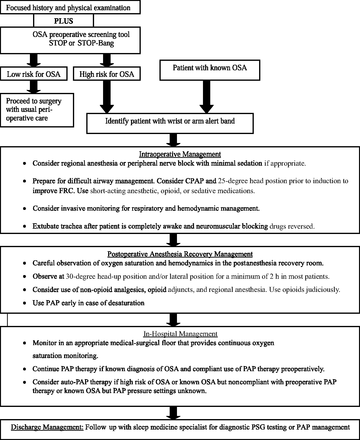
Adult patients deemed as having a high probability of OSA should be managed according to ASA guidelines7 (Figure 1). The surgical and anesthesia team need to be notified so perioperative management can be considered, including the use of alternatives to general anesthesia, such as regional or peripheral blocks, short-acting opioids, and multimodal analgesia (nonsteroidal anti-inflammatory drugs, acetaminophen, tramadol, ketamine, gabapentin, pregabalin, dexamethasone).62,63 Patients designated for same-day discharge should be observed for an extended period (generally up to 3 hours) in the recovery area.64 Oxygen desaturation (<94%, or ≥4% from baseline) during monitoring should be recorded and any apneas documented. If desaturation or apneas are noted and persist within 2 hours of the planned discharge time, then the patient should be admitted to the hospital for overnight observation. In addition, changing a patient's planned discharge from the same day to overnight observation should be considered for patients who require narcotic pain management.
Perioperative management of adult patients with obstructive sleep apnea (OSA) or at high risk for OSA undergoing elective, non–upper airway surgery. CPAP, continuous positive airway pressure; FRC, functional residual capacity; PAP, positive airway pressure; PSG, polysomnography. Adapted from Adesanya et al.5
For patients being admitted, pain management restrictions should include patient-controlled anesthesia for narcotic administration, no scheduled intravenous push narcotics, and avoidance of intravenous push hydromorphone (Dilaudid; Perdue Pharma, Stamford, CT). Hydromorphone is highly potent (1 mg of hydromorphone is equivalent to 8 to 10 mg of morphine), and many physicians underestimate the severity of its respiratory depressant effects. A retrospective analysis of 32 cases of respiratory failure at 1 medical center found that 3 of 4 deaths were associated with hydromorphone as the primary parenteral opioid.28 For this reason, morphine sulfate is preferred over hydromorphone for parenteral analgesia. Codeine should be avoided, especially in children, because its CYP2D6 metabolism to the active drug (morphine) is inconsistent, sometimes causing it to be ineffective in poor metabolizers, and occasionally leading to morphine overdose and death in extensive metabolizers.65,66
Patients with OSA should have continuous pulse oximetry monitoring with audible alarms for desaturation below 90% partial pressure of oxygen. Patients should preferably be within the line of sight of the nursing station.67 Supplemental oxygen should have a stop order set at 2 L/min (ie, the nurse must contact a physician to titrate the oxygen above 2 L/min). This is because the central response to hypoxia is reduced and the hypercapnic response is markedly reduced during REM sleep. Therefore excess oxygen could induce hypoventilation/hypercapnia by suppressing the central hypoxia response and by raising oxygen concentrations; this could delay the recognition of a respiratory problem by pulse oximetry.67
If possible, patients should be positioned to sleep on their side, or in a semiupright or other nonsupine position, since the supine position aggravates OSA by increasing the ability of the pharynx to collapse.7,29 Continuous positive airway pressure therapy can be administered for patients suspected to have undiagnosed severe OSA. All patients identified as having a high probability for undiagnosed OSA (same-day surgery patients going home and patients discharged from an overnight stay) should receive discharge instructions for follow-up with a sleep medicine consultant and with educational information on OSA, as well as instructions to avoid risks associated with postoperative narcotic use for pain control. (See Figures 1 and 2 for perioperative management algorithms; Table 6 for SOR).
Post-adenotonsillectomy disposition of children with obstructive sleep apnea (OSA). *Comorbidities in children <3 years old include severe OSA documented by polysomnography (PSG), failure to thrive, obesity, cardiac involvement (right ventricular hypertrophy), Down syndrome, history of prematurity, craniofacial abnormalities, neuromuscular diseases, chronic lung disease, and sickle cell disease (comorbidities are taken from Table 3 in Ref. 65). AHI, Apnea-Hypopnea Index. From Patino et al.65
Recommendations
In our opinion, use of the STOP or STOP-Bang questionnaire should become part of every adult preoperative examination. All pediatric preoperative exams should include a question about snoring; the I'M SLEEPY questionnaire can be used to further screen children at high risk of OSA, although this questionnaire requires further validation. The surgical team should be made aware of patients identified as having a high risk of OSA. When time permits during the period before surgery, high-risk patients should have a consultation with a sleep specialist and a home screening sleep study or complete polysomnography ordered when practical. Family physicians involved with the care of surgical inpatients should be aggressive about checking postoperative orders to ensure that patients at high risk of OSA have, at a minimum, continuous pulse oximetry monitoring with an alarm as a precaution against unobserved respiratory failure. Follow-up for OSA treatment after discharge is also essential for patients newly diagnosed through the screening process.
Finally, hospitals need to have standardized practice guidelines or order sets to guide the management of surgical patients with OSA. A U.S. survey (783 responses) found that only 27 % of respondents reported that their institution had a written policy for the perioperative care of patients with OSA, with the highest awareness among sleep medicine physicians (35% reported having such a policy). Only 6 % of internal medicine and family medicine physicians surveyed acknowledged having such a policy.68 A Canadian survey (773 responses) found that 53% of respondents indicated a policy was present in their institution; 34% of the respondents specified that no policy was present, and 13% did not know.69 Thus it seems likely that almost half of Canadian and perhaps two thirds of American healthcare institutions do not have policies on this topic; certainly family physicians and internists are mostly unaware of these policies if they do exist. Family physicians have an opportunity, through their involvement with their local hospitals and hospital committees, to be leaders in helping to implement policy standards for surgical patients with OSA.
Acknowledgments
The authors wish to acknowledge Neil S. Freedman, MD, Department of Pulmonary and Critical Care Medicine, NorthShore University HealthSystem, Evanston, IL, for helpful suggestions regarding postoperative care of the OSA patient.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
- Received for publication March 1, 2015.
- Revision received August 16, 2015.
- Accepted for publication August 19, 2015.