Abstract
Eosinophilic gastrointestinal disorders or eosinophilic digestive disorders encompass a spectrum of rare gastrointestinal disorders that includes eosinophilic esophagitis, eosinophilic gastroenteritis, and eosinophilic colitis. Eosinophilic gastroenteritis is a rare inflammatory disease characterized by eosinophilic infiltration of the gastrointestinal tract. The clinical manifestations include anemia, dyspepsia, and diarrhea. Endoscopy with biopsy showing histologic evidence of eosinophilic infiltration is considered definitive for diagnosis. Corticosteroid therapy, food allergen testing, elimination diets, and elemental diets are considered effective treatments for eosinophilic gastroenteritis. The treatment and prognosis of eosinophilic gastroenteritis is determined by the severity of the clinical manifestations. We describe a 24-year-old woman with eosinophilic gastroenteritis presenting as epigastric pain with a history of severe iron deficiency anemia, asthma, eczema, and allergic rhinitis, and we review the literature regarding presentation, diagnostic testing, pathophysiology, predisposing factors, and treatment recommendations.
A 24-year-old nulliparous African-American woman was admitted after an episode of near syncope associated with 2 days of fatigue and dizziness. She reported gradual onset of dyspepsia over 2 to 3 months. She denied nausea, vomiting, menorrhagia, polymenorrhea, diarrhea, hematemesis, melena, or hematochezia. Her medical history was significant for allergic rhinitis, eczema, and asthma. Her surgical history was negative. She denied using tobacco or illicit drugs. She drank approximately 1 to 2 beers per month. Her family history was significant for a father with asthma and hypertension and a mother with hypertension. Her medications included inhaled fluticasone, an albuterol metered-dose inhaler, a Flovent inhaler, loratadine, and ranitidine.
During examination, her height was 62 inches, weight 117 lb, and body mass index 21.44 kg/m2. Her heart rate was 111 beats per minute, blood pressure 121/57 mm Hg, respiratory rate 20 breaths per minute, and oral temperature of 98.6°F. She appeared acutely ill and lethargic. She was oriented to person, place, and time. She had pallor of the conjunctiva. Cardiovascular, respiratory, musculoskeletal, and neurological examinations were normal. Her abdomen was soft and nontender, without organomegaly. Rectal examination revealed brown stool that was hemoccult negative.
Initial laboratory tests revealed a hemoglobin level of 5.1 g/dL (normal range, 12.0–16.0 g/dL), a hematocrit level of 17.1% (normal, 37.0% to 47.0%), a mean corpuscular volume of 66.9 fL (normal, 80.0–98.0 fL), a red blood cell distribution width of 31.1%F (normal, 11.5–18.0%F), and a platelet count of 921,000/mm3 (normal, 150,000–400,000/mm3). The white blood cell differential included 82% neutrophils, 5% lymphocytes, 5% monocytes, 6% eosinophils, and 2% basophils. Human chorionic gonadotropin test was negative. Ferritin level was 2.3 ng/mL (normal, 10.00–291.00 ng/mL), iron level was 11 mcg/dL (normal, 50–175 μg/dL), and total iron-binding capacity was 404 μg/dL (normal, 250–450 μg/dL). International normalized ratio was 1.0. She was admitted for symptomatic anemia and transfused with 4 U packed red blood cells. After the transfusion, her hemoglobin level was 12 g/dL. The patient was discharged in stable condition and was seen 1 week later by a hematologist, who confirmed the diagnosis of severe iron-deficiency anemia, and oral iron replacement therapy was initiated.
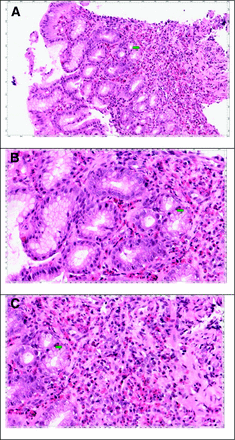
The patient returned 1 month later, complaining of epigastric pain, nausea, vomiting, and diarrhea. She reported several days of watery diarrhea and complained of anorexia. She reported one episode of hematemesis. Stool samples were sent for culture, gram stain, and examination for ova and parasites, and the stool studies were negative. After several days, abdominal pain persisted and omeprazole was added to the treatment regimen. An esophagogastroduodenoscopy was performed, which showed diffusely erythematous mucosa with an atrophic and nodular gastric mucosa extending into the first portion of the duodenum. Gastric biopsies were obtained for Helicobacter pylori and a pathology review. Omeprazole and ranitidine were continued. Gastric and duodenal biopsy results demonstrated diffuse glandular atrophy with dense eosinophilic infiltration (Figure 1). Pathology results were not consistent with celiac disease. She began taking oral prednisone with a slow taper over 6 weeks. A computed axial tomography scan of the abdomen and pelvis was obtained and was normal, without evidence of obstruction. She was referred to allergy/immunology. Subsequent immunoglobulin testing revealed a total immunoglobulin (Ig) E level of 1997 kU/L (reference range, 0.00–148.00 kU/L). Specific IgE subset testing showed elevated levels of IgE to eggs, milk, corn, oats, rice, wheat, peanuts, and soybeans. She began an elimination diet based on IgE testing but was noncompliant. She followed up after completing the course of prednisone, and her abdominal pain, diarrhea, nausea, and vomiting had completely resolved. Her hemoglobin remained stable.
Histology of gastric biopsies showing eosinophilic infiltration. A: The biopsy consists of gastric antral type mucosa that is heavily infiltrated by eosinophils. B: Many high-power fields contain more than 50 eosinophils. In addition, eosinophils infiltrate the glandular epithelium, and many of the glands appear damaged and exhibit reactive atypia. C: In addition, there appears to be loss of a significant number of glands. This may explain the atrophic appearance noted during endoscopic examination. The histologic features are characteristic of eosinophilic gastroenteritis.
Discussion
Eosinophilic gastrointestinal disorders or eosinophilic digestive disorders (EGIDs) encompass a spectrum of rare gastrointestinal disorders that includes eosinophilic esophagitis, eosinophilic gastroenteritis, and eosinophilic colitis.1 These conditions are increasingly being recognized despite the varying clinical presentation, likely as a result of increased diagnostic testing with esophagogastroduodenoscopy and colonoscopy, both with biopsy. Under normal, nonpathologic conditions, the gastrointestinal tract is the only nonhematopoietic organ to contain eosinophils, and the cecal and appendiceal regions have the highest concentrations.2 It is postulated that exposure of the gastrointestinal mucosa to antigens promotes a Th-2-mediated immune response.3 Th-2 cells produce interleukin (IL)-4, IL-5 and IL-3 and promote the production of eosinophils as well as IgE.3
Eosinophilic esophagitis is the most commonly recognized EGID; most often it is seen in white men and presents with symptoms of gastroesophageal reflux disease. Eosinophilic gastroenteritis is a condition in which eosinophils infiltrate either the stomach or small bowel, and approximately 50% may have atopic disorders, food intolerances, or food allergies.4 Eosinophilic colitis is a rare condition that causes colonic eosinophilia, and patients typically present with abdominal pain, diarrhea, and weight loss.4 The clinical manifestations of EGIDs depend on which layer and which bowel segments are predominantly involved. EGID is classified into mucosal, submucosal, and serosal disease5 (see Table 1). Except for the serosal form, in which 75% of the patients are women aged 40 years or older, eosinophilic gastroenteritis is a predominantly male disorder that affects children as well as adults.10
Literature Search Strategy
We searched PubMed, Ovid, and Web of Knowledge using search terms eosinophilic gastroenteritis and eosinophilic gastrointestinal disorders but excluded the search terms eosinophilic esophagitis, eosinophilic colitis, and allergic gastroenteropathy. We found no randomized controlled trials or systematic review articles but found approximately 300 case reports and case series.
Clinical Symptoms
The most common symptoms of eosinophilic gastroenteritis include abdominal pain (90%), diarrhea (60%), vomiting (60%), nausea (50%), and abdominal distension (50%).11,12 Eosinophilic gastroenteritis affects all races and age groups, although in adults it usually presents during the third to fifth decades of life.13 It is necessary to rule out other causes of eosinophilia, such as drug reaction, parasitic infection, and cancer.14 The differential diagnosis of eosinophilic gastroenteritis includes disorders of eosinophilic infiltration, collagen vascular disorders, inflammatory bowel disease, malignancies, medications (eg, enalapril), and hypereosinophilic syndrome (Table 2).
Diagnostic Testing
A clinical history of other atopic conditions such as asthma, atopic dermatitis, and food and environmental allergies should heighten suspicion for eosinophilic gastroenteritis. Peripheral eosinophilia and elevated IgE are suggestive findings of initial laboratory testing.3,15 Additional testing may include allergy evaluation by skin and serum radioallergosorbent tests, albumin and protein levels, α-1 antitrypsin levels, and analysis of ascitic fluid for eosinophils.1
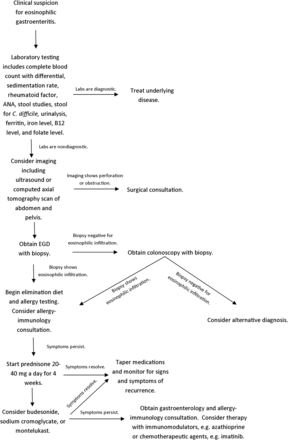
Radiologic tests are nonspecific but may show varying degrees of inflammation such as mucosal thickening and irregularity. The most common computed tomography finding is nodular and irregular folds and thickening in the distal stomach and proximal small bowel.16 Abdominal ultrasound may detect ascites. Definitive diagnosis requires esophagogastroduodenoscopy with biopsy showing histologic evidence of eosinophilic infiltration (see Figure 2).
Algorithm for the workup and treatment of eosinophilic gastroenteritis. ANA, antinuclear antibody.
Therapeutic Options
Food allergy testing and elimination and elemental diets should be considered and often are performed before a trial of corticosteroids. Spontaneous remission may occur in up to 40% of patients.17 In pediatric cases of eosinophilic gastroenteritis, elimination diets have been beneficial, in some cases without a need for additional treatment, and may describe a subset of patients with allergic eosinophilic gastroenteritis.7,18,19 Corticosteroids are the mainstay of therapy, and most patients have symptomatic response. Symptom relief usually occurs within a few weeks of the initiation of treatment.16,20 Treatment with histamine H-1 receptor antagonists or mast cell–stabilizing drugs (eg, cromoglycate), has been described in previous studies and may be considered in patients who do not respond to or cannot tolerate corticosteroids. Montelukast, a selective and competitive leukotriene receptor antagonist, may be considered as a corticosteroid-sparing agent.16,21 Consultation with an allergist/immunologist or a gastroenterologist should be considered for patients with persistent symptoms despite an adequate trial of corticosteroids. Therapy with immunomodulators (eg, azathioprine) or chemotherapeutic agents (eg, imatinib) may be considered in patients with severe and unremitting symptoms. The natural course of the disease is uncertain and relapses are frequent; therefore, patients should be monitored after therapy has been initiated to reassess for symptom recurrence or complications. Surgery usually is not necessary but is sometimes needed in cases of intestinal obstruction or perforation.
Conclusions
Additional studies are needed to follow the natural history of this disorder. Randomized, control trials are needed to assess the effectiveness and safety of pharmacological and nonpharmacological therapies for eosinophilic gastroenteritis. Eosinophilic gastroenteritis is a chronic gastrointestinal disease with a variety of clinical presentations. A high degree of clinical suspicion is needed for accurate diagnosis. Oral corticosteroids are the mainstay of therapy.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
- Received for publication September 14, 2011.
- Revision received December 12, 2011.
- Accepted for publication December 19, 2011.