Abstract
Background: Urban minority groups, such as those living in northern Manhattan and the South Bronx, are generally underserved with regard to breast cancer prevention and screening practices. Primary care physicians are critical for the recommendation of mammography and clinical breast examinations to their patients.
Design: Two medically underserved communities were matched and block randomized. The aim of the study was to assess the efficacy of academic detailing in increasing recommendations for breast cancer screening in community-based primary care physicians.
Setting/Participants: Ninety-four primary care community-based (ie, not hospital-based) physicians in northern Manhattan were compared with 74 physicians in the South Bronx who received no intervention.
Intervention: Intervention participants received multicomponent physician-directed education, academic detailing, using the American Cancer Society guidelines for the early detection of breast cancer.
Main Outcome Measures: We administered interviews to ask about primary care physicians’ recommendation of mammography and clinical breast examination. They were also queried about their knowledge of major risk factors and perceived barriers to breast cancer screening. We conducted medical audits of 710 medical charts 2 years before and after the intervention.
Results: Using a mixed models linear analysis, we found a statistically significant intervention effect on the recommendation of mammography and clinical breast examination (according to medical audit) by female patients age 40 and over. Intervention group physicians correctly identified significantly more risk factors for breast cancer, and significantly fewer barriers to practice, than did comparison physicians.
Conclusions: We found some evidence of improvement in breast cancer screening practices due to academic detailing among primary care physicians practicing in urban underserved communities.
Although the use of breast cancer screening has risen considerably over the past 10 years, the rates among African American and Hispanic women still fall behind those of white women.1 This difference is due, in part, to variations in physician screening behaviors,2 particularly among underserved, low income patients.3 Disparities in screening contribute to increased morbidity and age-adjusted mortality from breast cancer among African American women relative to non-Hispanic whites.4–8
Studies have found that clinical practice guidelines have had limited success in influencing physician behaviors.9–13 But, two recent meta-analyses have highlighted the importance of physician recommendation to reducing these disparities.2,14
Academic detailing entails a brief face-to-face intervention with the physician, repeated at periodic intervals. Detailers also share materials and approaches that are tailored to the physician’s barriers to screening. Traditionally employed by pharmaceutical companies to promote prescription drug uptake among physicians, academic detailing has been found to be effective in many studies in which it has been evaluated.15–26
Some of these studies focus exclusively on prescribing (13 of the 18 studies cited by Hulscher et al27), have negative findings,28,29 or are inconclusive.30,31 These mixed findings highlight the need for more study of the impact of academic detailing for preventive services, particularly among medically underserved populations.
Academic detailing, as a multicomponent intervention,31,32 also includes techniques and tools that address office-based barriers to screening. Physician reminders (eg, chart flags, manual or computerized reminder systems33–36) and multilingual, low literacy patient education materials,37 among other components, have demonstrated increased breast,22 cervical,38 and colorectal39,40 cancer screening in physicians’ practices.
The intervention, academic detailing, relies on constructs from well-established theories to increase physician behavioral change.41 The theory of planned behavior (TPB)42 posits the influence of focused attitudes and beliefs and social norms on breast cancer screening recommendation. By placing great importance on decision making, the TPB attempts to predict behaviors not entirely under individual control. Social cognitive theory (SCT)43 postulates that persons with high levels of self-efficacy and beliefs that positive outcomes will derive from screening will be most likely to recommend it. In SCT, social norms influence both cognitions and behavior. Fundamentally, academic detailing seeks to change physicians’ attitudes and beliefs toward screening through persuasive communications, and to alter their cognitions through tailored feedback and reinforcement. Concomitantly, a prevention-oriented office context (eg, through trained staff) and cues in office procedures (eg, flagged medical charts) enrich the physician’s memory for new information and reinforce behavioral patterns.44
The objective of this study was to assess the efficacy of academic detailing in increasing recommendations for breast cancer screening in a sample of community-based urban physicians compared with physicians in a similar community. To date, there have been no reported studies of the use of academic detailing as a method for increasing adherence to breast cancer screening guidelines among medically underserved African American and Hispanic populations.
Subjects and Methods
We matched the northern Manhattan (Harlem and Washington Heights) and South Bronx communities, areas with higher mortality from breast and other cancers,27,45,46 using US Census data, by the percentage of minority residents and those in poverty. These communities have been compared with a developing country,47 with residents who are generally poor (median household income = $23,656 per year), with less education (15% hold a bachelor’s degree or higher), and primarily African American (33%) or Hispanic (54%). Approximately one-third (31%) of the residents of these communities are foreign born (primarily from Latin America or the Caribbean/West Indies); 43% speak only English at home, illustrative of their recent immigration to the United States. Only 51% of their residents are employed; of those who are working, approximately one-quarter (26%) hold managerial or professional positions. Of residents age 65 and older, 58% are disabled. Families in these communities have large families (median size, 3.4 persons).
We block randomized 48 of the physicians in the northern Manhattan community to the intervention condition, and those in the South Bronx to the comparison arm. We used block randomization at the level of the community to reduce clustering due to posited similarities among local primary care providers, and to decrease possible spillover effects between intervention and comparison physicians within these contiguous geographic areas.
Participants
To identify physicians working in northern Manhattan and the South Bronx, we collected licensing lists from New York State, directories from local hospitals, and names from our physician advisory board. We conducted door-to-door surveys of these communities to identify any additional physicians’ offices. Of approximately 642 physicians in these communities who were contacted by telephone to assess eligibility, 359 devoted at least 50% of their practice to primary care, were community-based (ie, not hospital-based), and were not expecting to leave the area over the coming year, so met the study criteria. We enrolled 192 (53%) of these physicians at baseline with a verbal consent. As is common in studies of organizations,49 we enrolled only the most senior fulltime (and thus the most influential) physician in the office. Physicians received Continuing Medical Education credits for their participation.
Both physician groups completed a baseline and a 12-month follow-up to assess changes in breast cancer knowledge, perceived barriers to screening, and the practice of early detection behaviors.
Of 192 eligible physicians, 87% completed both a baseline and follow-up, yielding a final sample of 168 (94 intervention and 74 comparison physicians’ offices). Two physicians retired, 5 moved, and 17 no longer practiced primary care, became too ill to practice, or refused to participate in the follow-up (N = 24). The study was approved by the Institutional Review Board of Columbia University.
Measures
The 57-item questionnaire, administered in face-to-face interviews by project staff in 1997 to 1998 and 2001 to 2002, contained self-report items50,51 that assessed the physician’s sociodemographic and medical practice characteristics, breast cancer prevention knowledge, attitudes/beliefs, and practices. Physicians’ estimates of breast cancer screening practices were based on the binary answer (yes/no) to the following questions about mammography and clinical breast examination (CBE; by a health care provider): whether the physician conducts or recommends the procedure; if yes, the frequency of those screenings for asymptomatic women age 40 to 49, and age 50 and over. One item assessed the recommendation of breast self-examination (BSE) by the patient. Physicians were asked about the perceived barriers to breast cancer screening, using 8 items,27,52 including; no medical indication, low yield, risk of radiation, resistance by patients, cost of the test, causing unnecessary worry for patients, the risk of false positives, and other (ie, patient discomfort, patient pain, prefer female provider, frequent lumps). The number of barriers was summed. To examine physicians’ knowledge of other breast cancer prevention approaches, they were asked approximately 10 common risk factors, including: positive family history for breast cancer, for ovarian cancer, for breast and ovarian cancer; personal history of breast cancer, of ovarian cancer, of breast and ovarian cancer; increased age, late age at first pregnancy or nulliparity, early age at menarche, late age at menopause.53–58 The number of correctly identified risk factors were summed to form a continuous measure.
Physician’s self-reported age, number of years of medical practice, number of patient contacts per week, percentage of patients in the medical practice among different ethnic/racial and insurance subgroups (including the uninsured) were measured as continuous variables. Primary care physician’s (PCP) graduation from a US or foreign medical school and race/ethnicity were categorical measures. These sociodemographic and medical practice measures have been associated with physician cancer screening behaviors.31,41
To measure the implementation process for office-based breast cancer prevention at follow-up, we administered a 12-item subscale.49,50 The items were counted to create one “implementation score” per physician.
The instrument was pilot-tested on primary care physicians who were not included in the final study. Subscale items exploring the perceived barriers to cancer prevention and screening were moderately internally consistent (Cronbach’s α = 0.60 to 0.73).
Audits were conducted on 710 charts at follow-up by 5 trained research assistants who were supervised by one of us (SSG), among a randomly selected subset of 15% of the physicians. We conducted medical audits on 13 offices each in the intervention and comparison groups, to reduce participant burden. Within the offices, using a table of random numbers, the trained abstractors who were blind to intervention group status, selected a sample of medical charts of women aged 40 and over who had not been diagnosed with breast cancer, with at least one documented visit to the physician over the past 2 years (median = 46 charts). Each chart was abstracted, using a structured form (available from SSG), the 2 years before and after the intervention. Radiologist’s reports of all mammograms were abstracted. We defined a recommendation for mammography and/or CBE either explicitly, with a notation in the chart (ie, a written referral for mammography, conduct of a CBE or a mammogram by a health care provider or from another screening center), or implicitly, with a radiologist’s report of the findings from a mammogram.61 A mammogram or CBE was characterized as screening if the physician recorded “screening” as the purpose of the procedure, noted it on the referral, or if there were no relevant patient symptoms (eg, pain, nipple discharge) in the medical record at the time of the recommendation.
Academic Detailing Intervention
Ninety seven percent of the intervention physicians received 4 academic detailing visits with self-learning packets (ie, professionally designed print materials, scientific articles, and a targeted verbal script) from 2 Master’s level health educators; the remaining 3 physicians received 3 face-to-face visits each over a 2-year period of time. Throughout, we highlighted the American Cancer Society (ACS) breast cancer screening recommendations for asymptomatic women, age 40 and over, as they are the most widely recognized guidelines in these local practices. At the time, the ACS recommendations included mammograms every year for women age 40 and older, CBEs for women ages 20 to 40 every 3 years, and CBEs every year for women age 40 and over. For women age 20 and over, the ACS recommended a monthly breast self-examination (BSE).
Academic detailing contacts with the physician were brief (average, 9.25 minutes). If the physician consented, the office-based breast cancer prevention materials (adapted from Ref. 59) were shared with the other staff as well.
To increase efficient contact with the intervention physicians, visits were supplemented by 6 dinner seminars; 46% of the intervention physicians attended. We also disseminated a newsletter to decrease attrition.
Before implementation, the academic detailing script and print materials were evaluated for face validity by a panel of physicians who served on an advisory board. For patient education, we used multilingual American Cancer Society materials that have been evaluated for readability.62
Analytic Plan
Differences between the sociodemographic, knowledge, attitude/belief, and screening factors by condition were each tested via χ2 analyses (or Mantel Haentzel X2 for screening recommendations) or by an analysis of variance (ANOVA). The independent factors were further evaluated as confounds; those items found to be statistically significant (P < .05), and interaction terms, were tested simultaneously for their effects on the rates of screening. The number of years the physician had been in practice was included in all multivariate models a priori.
As the knowledge of risk factors and barriers to screening were collected at the physician level, and were continuous measures, they were analyzed via a hierarchical multiple linear regression analysis, with the intervention term entered last. Standard diagnostic techniques were used to test for multicollinearity and the model performed favorably; similarly; a plot of the residuals revealed no problematic patterns.
Physicians’ self-report data tend to over-estimate their screening behaviors.61,63–65 Therefore, we used the medical audit data to calculate the major study outcome, measured as the proportion of screened women to all women (age 40 and over) per physician. All multivariate analyses of the medical audit data, calculated as proportions, were conducted at the patient level using the mixed effects model GLIMMIX in SAS,66,67 with a random physician effect. This model was selected to account for clustering among patients within physicians’ offices, improve power, enhance the flexibility to examine some patient-level covariates, and because of clinical interest. Goodness-of-fit statistics were examined to determine the adequacy of the model.
Missing data for the practice measures (<5%) were imputed by the researchers with the mean value.68 When applicable, all p-values resulted from use of 2-sided tests.
Results
At baseline, the intervention (N = 94) and comparison (N = 74) physicians were similar by age, gender, race/ethnicity, number of years of practice, practice size (in number of patient contacts per week), patient racial/ethnic characteristics and the implementation score; fewer physicians in the comparison group graduated from an American medical school (P < .0001), practiced with patients who have Medicaid and Medicare coverage (P < .0001), and had managed care contracts (P < .001) than physicians in the intervention group (see Table 1). More physicians in the comparison group saw patients with “other” payer sources (eg, self-pay) than intervention participants (P < .0001). These sociodemographic and medical practice baseline differences were included as confounders in the multivariate analyses.
Sociodemographic and medical practice characteristics of the primary care physician sample at baseline (N = 168)
Analyses of Physician Self-reported Knowledge of Risk Factors for Breast Cancer and Barriers to Screening
Multiple linear regression analyses were conducted to evaluate the impact of the intervention on PCPs’ knowledge of breast cancer risk factors and perceived barriers to breast cancer screening at follow-up relative to baseline (see Table 2). Covariates included whether the PCP attended a US (or foreign) medical school, knowledge of breast cancer risk factors, perceived barriers to breast cancer screening, number of years of practice, percentage of patients enrolled in managed care, percentage of patients enrolled in other insurance, and percentage of patients enrolled in Medicare or Medicaid.
Multiple linear regression analysis of the effect of academic detailing on (1) knowledge of risk factors at post-test and (2) barriers to breast cancer screening (N = 168 for both)*
The intervention group reported significantly more knowledge of breast cancer risk factors at follow-up than at baseline, compared with the physicians in the control group (P < .01, see Table 2). Physicians in practices with more patients who received Medicare or Medicaid were less influenced in their knowledge of risk factors for breast cancer by the intervention than other PCPs (P < .04). PCPs who could name more relevant risk factors at baseline could also identify more at follow-up (P < .00001). The intervention group perceived fewer barriers to the practice of breast cancer screening at follow-up than at baseline, compared with physicians in the control group (P < .00001, see Table 2). PCPs who identified more barriers at baseline also identified more at follow-up (P < .0001). These effects remained after the introduction of confounders.
Medical Audit Findings on Breast Cancer Screening
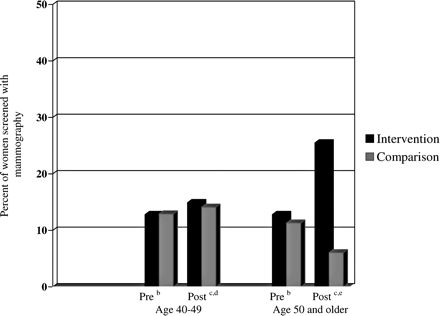
Repeated measures ANOVA analyses of the medical audit data on the use of mammography revealed a significant intervention effect for women age 40 and older compared with the control group (see Figure 1). There was no intervention effect for CBE recommendations to women age 40 and older (see Figure 2). Overall, we recorded only 16 chart notations for teaching or recommending BSE; these data were too few to analyze.
Effect of academic detailing on percentage of women recommended for screening with mammography using chart audit data: b, 2 years preintervention; c, at follow-up after completion of academic detailing intervention; d, repeated measures ANOVA (P = .01); e, repeated measures ANOVA (P = .01).
Effect of academic detailing on percentage of women recommended for screening with clinical breast examination (CBE) using chart audit data: b, 2 years preintervention; c, at follow-up after completion of academic detailing intervention; d, repeated measures ANOVA (P = .98); e, repeated measures ANOVA (P = .95).
Linear Mixed Model Analyses of Medical Audit Data on Screening Recommendations
As patients were clustered within physician practices, linear mixed models with the physician as a random effect examined the relationships between the intervention and the comparison groups on the proportion of women recommended for screening over the past 2 years using medical audit data (see Table 3). Covariates in the model included whether the PCP attended a US (or foreign) medical school, number of years of practice, baseline rates of mammography or CBE as appropriate to the outcome, follow-up knowledge of breast cancer risk factors, follow-up barriers to breast cancer screening, percentage of patients enrolled in managed care, percentage of patients enrolled in other insurance, and percentage of patients enrolled in Medicare or Medicaid.
Linear mixed models analysis of the effect of academic detailing on physician recommendations for (1) mammography at follow-up and (2) clinical breast examination (CBE) at follow-up (N = 710 for both)*
PCPs who were assigned to the intervention condition were more likely to recommend mammograms to women age 40 and older at follow-up than control physicians (P < .002; see Table 3). PCPs who recommended mammograms before the intervention were significantly more likely to recommend them at follow-up than were comparable physicians (P < .0001). The smaller the percentage of women in a PCP’s practice with Medicaid or Medicare, the more mammograms the physician recommended at follow-up (P < .0001). Finally, the greater the PCP’s knowledge of risk factors for breast cancer, the greater proportion of women age 40 and older recommended for mammography screening (P < .0001). The covariates of number of years of practice (for CBE), percentage of patients in managed care, and the barriers to practice were deleted from the model because their contributions were null.
Similarly, PCPs who were assigned to the intervention condition were twice as likely to recommend CBEs to women age 40 and older at follow-up than were comparison physicians (P < .002; see Table 3). The smaller the percentage of women in a PCP’s practice with Medicaid and Medicare relative to other insured women, the more CBEs the physician recommended at follow-up (P < .0001). As with the findings for mammography recommendations, the greater the PCP’s knowledge of risk factors for breast cancer, the greater proportion of women age 40 and older recommended for CBE (P < .0001). There was also a statistical trend toward more recommendations for CBE among PCPs who identified fewer barriers to screening than among comparable physicians (P < .06). None of the other measured factors were associated with PCPs’ CBE recommendations. The managed care factor was deleted from the model because of a null contribution. There were no statistically significant interactions in any of the tested models.
Discussion
Academic detailing seemed to increase primary care physicians’ recommendations for mammography and CBE among women age 40 and older. The findings are strong and consistent using medical audit measures for mammography and CBE among women age 40 and older. The findings on the impact of academic detailing on preventive behavior among PCPs are also consonant with those from a recent randomized clinical trial26that successfully increased recommendations for smoking cessation counseling among primary care physicians; the preventive aim, the modification of counseling skills, the sample of community-based physicians, and patient verification are notably similar, suggesting the strength of the result. The findings on increased CBE recommendations post-intervention are consistent with those from another study of the impact of physician office-based education on CBE for breast cancer screening,18 despite differences in the patient populations. The consistency of these results across several studies suggests robust findings.
The rates of mammography at follow-up are consistent with those from the National Health Interview Survey (NHIS) for African American and Hispanic women who dominate in these communities.69 CBE rates are still below population-based comparisons from patients’ self-report,1,70 however, perhaps due to under-notation in the medical record and some (9% in our sample) referral to obstetricians and gynecologists for the procedure. As providers generally receive no additional payment for performing the CBE,71 and institutional performance standards for CBE are inconsistent,72 there are few incentives for the physician to record the activity in the medical record. Further, perhaps Medicaid or Medicare coverage influences the likelihood of PCP’s breast cancer screening.
The intervention physicians correctly reported more risk factors for breast cancer, and fewer barriers to the practice of breast cancer screening than comparison physicians. Physicians’ familiarity with screening guidelines, and their attitudes and beliefs toward testing seem critical to explaining their compliance with professional recommendations.73–76 Separate analyses of the self-report data revealed that the intervention reduces perceived patient resistance to, cost of, and worry about screening, even though the scientific controversies that surround mammography (ie, low yield, risks of false positives) remain barriers to physician screening recommendations. We found that office-based procedures (for review, see Ref. 32), although more common among intervention than comparison participants at follow-up, may be necessary, but not sufficient to effect breast cancer screening changes in these underserved communities.
Interestingly, the comparison physicians also increased their mammography screening recommendations to all eligible women from baseline to follow-up. These increases may reflect the effect of study participation that sensitized control PCPs to breast cancer screening. These increases may also reflect the burgeoning interest in breast cancer developed by advocacy groups,77 and both provider and patient attention to the national controversies about breast cancer screening78 during the intervention years of the late 1990s.
Academic detailing is a moderate cost intervention (approximately $721.77 per participant79); as a result, one large department of public health (in New York City) has implemented the intervention for several prevention approaches among primary care physicians city-wide. Nonetheless, the intervention may not be fiscally feasible in other communities. Through cooperative agreements, however, departments of public health may encourage pharmaceutical and insurance companies to incorporate health promotion messages into their existing detailing practices.
Although the study has strengths, it also has several limitations. The study used block randomization at the level of the community; analyses were conducted among physicians and patients; despite the acceptance of this design approach for prevention trials (eg, Collaborative group for COMMIT trials80) and the application of appropriate statistical approaches to the data analysis, unmeasured differences may have influenced outcomes. We obtained relatively high rates of physician study participation (comparable with Myers et al81 and higher than the 21% enrollment obtained among health plan-affiliated provider organizations to participate in a similar study of colorectal cancer screening by Ganz et al82), and audited the medical record to obtain the rates of physician screening recommendations. Further, chart audits demonstrate superior accuracy in capturing physician screening behaviors relative to self-report.83,84 Four sociodemographic or medical practice factors differentiated the intervention and control PCPs at baseline; they (notably, Medicare or Medicaid coverage, as mentioned earlier) may have confounded the relationship between study arm and CBE screening, in particular. These factors were included as covariates in all the multivariate analyses, and comparable testing was conducted in both groups, so that their impact on the outcomes could be examined directly, however. To reduce the respondent burden on these often turbulent urban offices, we collected medical audit data from a small subsample of physicians. The size of the audited subsample may limit the generalizability of the results. The audited offices were selected at random, and the rates for mammography are consonant with those found in a population-based household survey of the community, however.65,70 Although we used well-trained, blinded, closely supervised medical abstractors, they may have surmised the intervention group, thus biasing the findings.
The study suggests that this clinically based intervention, academic detailing, may increase screening recommendations among urban practices that are dominated by Hispanic and African American women, who are themselves more likely to die of breast cancer than are other sub-populations.
Acknowledgments
We thank Stefanie Jean Baptiste for assistance with data collection. We are grateful to Dr. Alfred I. Neugut for comments on an earlier version of the paper.
Notes
Funding: This study was funded by the National Cancer Institute (R25 CA66882).
An earlier version of this paper was presented in part at the Annual Meeting of the American Society of Preventive Oncology, 10 March 2002, in Bethesda, MD.
Conflict of interest: none declared.
AH is deceased.
- Received for publication November 23, 2004.
- Revision received September 17, 2005.
- Accepted for publication September 23, 2005.