Abstract
Background: Measurements of C-reactive protein (CRP) concentration and erythrocyte sedimentation rate (ESR) are frequently ordered jointly in clinical practice.
Aim: To investigate the factors associated with discordances between CRP concentration and ESR in adults.
Methods: We conducted a cross-sectional study of 1472 adults with no known inflammatory disorders (44.5% male; median age, 52 years; range, 18–91 years), randomly selected from a municipality in Spain. The participants underwent simultaneous measurements of ESR, serum CRP, and interleukin-6 concentrations. Alcohol consumption, smoking, and physical activity were evaluated by questionnaire. Body mass index (BMI) measurement and metabolic syndrome criteria were available for all participants.
Results: Most (n = 1123, 74.9%) of the participants showed normal CRP and ESR values. Sixty-nine (4.6%) participants showed high CRP and ESR values. Seventy-two (4.8%) participants showed a discordant pattern of high ESR and normal CRP values, which was associated with age after adjusting for sex, alcohol consumption, physical activity, BMI, and the presence of metabolic syndrome (odds ratio [OR], 1.052; 95% CI, 1.034–1.071; P < .001). A total of 208 (13.8%) participants showed a discordant pattern of high CRP and normal ESR values, which was associated with BMI after adjusting for covariates (OR, 1.099; 95% CI, 1.064–1.136; P < .001). BMI appeared to be the main determinant of serum CRP concentrations in this population. Serum interleukin-6 concentrations were positively associated with the discordant pattern of high CRP and normal ESR values.
Conclusion: In this general adult population with no overt inflammatory disease, the discordant pattern of high ESR and normal CRP was associated with greater age, whereas the pattern of high CRP and normal ESR was associated with higher BMI.
- Age
- Alcohol
- Body Mass Index
- C-Reactive Protein
- Erythrocyte Sedimentation Rate
- Inflammatory Disorders
- Interleukin-6
- Metabolic Syndrome
- Obesity
- Physical Exercise
- Sex
- Smoking
Introduction
Serum or plasma C-reactive protein (CRP) concentration and erythrocyte sedimentation rate (ESR) are employed in routine clinical practice as laboratory markers of systemic inflammation. CRP was first described in 1930 in a patient with pneumococcal pneumonia.1 It is synthesized by hepatocytes after stimulation by cytokines (particularly interleukin [IL]-6), which are released during infection and tissue inflammation.2⇓–4 CRP plays a role in the host defense and displays both inflammatory and anti-inflammatory actions.2,3 Serum CRP concentrations are elevated in a variety of inflammatory disorders of infectious and noninfectious causes, as well as in certain malignancies.5 The ESR was first described more than a century ago6 but is still widely employed.7⇓⇓–10 ESR is the rate (in mm/h) at which red blood cells develop aggregates11 and depends mostly on the concentration of circulating acute-phase proteins, specifically fibrinogen.8,11 Similar to CRP, the ESR can increase in a variety of inflammatory and neoplastic conditions.7⇓⇓–10
Measurements of CRP concentration and ESR are frequently ordered jointly in clinical practice12 and are intended to detect and monitor systemic inflammation; however, their results do not exactly correlate, and discordances can be present.12 The majority of previous studies that have investigated the agreement between CRP and ESR values have included selected samples of patients with specific inflammatory diseases.10,13⇓⇓⇓⇓⇓⇓–20 More general studies comparing ESR and CRP have included patients with various diseases in the hospital21⇓⇓⇓⇓–26 and primary care settings.27⇓–29 In general, CRP and ESR have similar diagnostic accuracy assessing acute inflammation,10 though CRP tends to be more reliable for disease monitoring because CRP levels change faster than the ESR12,26,27 and are less affected by patient age.27,29 In patients with chronic inflammatory conditions, CRP and ESR offer similar information,10,13⇓–15 though discordances can exist.16⇓⇓⇓–20 To the best of our knowledge, no study has investigated the potential factors associated with disagreement between CRP and ESR results in unselected samples from general adult populations with no apparent inflammatory disease, which is an important issue because CRP and ESR can be routinely ordered among blood screening tests for individuals who are asymptomatic, mildly symptomatic, or with unspecific clinical manifestations. Moreover, determining the distribution of CRP concentrations compared with ESR in general populations is important for interpreting reference values. The present study aimed to add information to the factors associated with inflammatory marker elevation by investigating the discordance between abnormal CRP and ESR values in the general adult population. We also investigated the correlation between serum IL-6 concentrations and both ESR and CRP abnormalities.
Methods
Study Population and Design
This cross-sectional study was conducted in the municipality of A-Estrada (Spain), as reported elsewhere.30 An outline of the study (A-Estrada Glycation and Inflammation Study [AEGIS]) is also available at www.clinicaltrials.gov (code NCT01796184). Briefly, the study included an age-stratified sample (n = 1516) of the adult population from the municipality. Seventeen individuals were excluded from the study due to specific inflammatory diseases. Of the remainder, 1472 participants (44.5% male; median age, 52 years; range, 18–91 years) underwent a simultaneous measurement of CRP and ESR (see below) and completed an interviewer-administered questionnaire at the primary care center. The study was approved by the Regional Ethics Committee (code 2010-315). We obtained written informed consent from all the participants.
Assessment of Lifestyle Variables
Alcohol Consumption
We evaluated the number of standard drinking units31 regularly consumed per week, totaling the number of glasses of wine (1 unit, approximately 10 g), bottles of beer (1 unit, approximately 10 g), and spirits (2 units, approximately 20 g) consumed. Participants with an alcohol consumption of 1 to 13 units/week, 14 to 27 units/week, ≥28 units/week were considered light, moderate, and heavy drinkers, respectively. The remainder, comprising alcohol abstainers and occasional alcohol drinkers, were grouped.
Smoking
We considered consumers of at least 1 cigarette per day to be smokers. Individuals who had quit smoking during the preceding year were still considered smokers, whereas those who had quit more than 1 year before the study were considered ex-smokers.
Physical Activity
All study participants completed the freely available International Physical Activity Questionnaire (short version, https://sites.google.com/site/theipaq/home), which has been validated in Spain.32 The questionnaire allows for the calculation of metabolic equivalents of task and for the stratification of habitual physical activity as low, moderate, or high,33 as previously described.30
Definition of Metabolic Abnormalities
Body mass index (BMI) was calculated as weight (in kg) divided by the square of height (in meters). Accordingly, we classified the participants as having normal weight (<25 kg/m2), overweight (25 to 30 kg/m2), or obesity (>30 kg/m2). We considered the participants as having metabolic syndrome if they had at least 3 of the following Adult Treatment Panel III criteria34: (1) abdominal obesity (waist circumference >102 cm for men and >88 cm for women); (2) hypertriglyceridemia (fasting serum triglycerides ≥150 mg/dL); (3) low high-density lipoprotein (HDL)-cholesterol levels (fasting HDL-cholesterol levels <40 mg/dL for men and <50 mg/dL for women); (4) increased blood pressure (arterial blood pressure ≥130/≥85 mmHg or current antihypertensive medication use); and (5) hyperglycemia (fasting serum glucose levels ≥110 mg/dL or current antidiabetic therapy).
C-Reactive Protein Assay
We measured wide-range CRP (wrCRP) concentrations in fresh serum samples using commercial latex-enhanced immunoturbidimetry in an Advia 2400 Clinical Chemistry System (Siemens, Germany). Serum C-reactive protein (CRP) causes agglutination of the latex particles coated with antihuman C-reactive protein. The agglutination of the latex particles is proportional to the CRP concentration and can be measured by turbidimetry. The reference interval (normal values) for adults employing this method is 0–0.5 mg/dL (0–5 mg/L). CRP measurements were available for 1499 participants.
Erythrocyte Sedimentation Rate Assay
We measured the ESR in blood drawn in vacuum tubes containing K3EDTA (Becton Dickinson, USA) employing an automated TEST-1 device (Alifax, Italy). TEST-1 has been validated using the reference Westergren method following the International Council for Standardization in Hematology criteria.35,36 The reference ESR values are 0–20 mm/h for men and 0–30 mm/h for women. The ESR was available for 1472 participants, and their results in this population have been reported elsewhere.30
Interleukin-6 Assay
We measured IL-6 concentrations in fresh serum samples using a commercial chemiluminescent immunoassay (IMMULITE 2000 System, Siemens). The sensitivity limit for this method is 2.0 pg/mL. According to the manufacturer, in reference range study for IMMULITE 2000 IL-6 on serum samples collected from 60 healthy laboratory volunteers, the nonparametric lower 95% range was from nondetectable to 3.4 pg/mL, and the absolute range was from nondetectable to 5.9 pg/mL. Interleukin-6 measurements were available for 1499 participants. The distribution and factors associated with serum IL-6 concentrations in this population have been reported elsewhere.37
Statistical Analyses
We used the Mann-Whitney test to compare numeric variables, and the Jonckheere-Terpstra trend test compares the numeric variables in ordinal categories. We used Spearman's rank test to assess the correlation and employed linear regression for multivariate analysis of the factors associated with CRP concentrations. For that purpose, we log10-transformed the CRP values to normalize their distribution. For the logarithmic transformation, we attributed an arbitrary value of 0.001 mg/dL for cases with undetectable CRP (n = 4). For the statistical calculations, we considered IL-6 concentrations below the detection level (n = 742) as 2 ng/mL. We defined groups were defined according to the normality or abnormality of CRP levels (>0.5 mg/dL [5 mg/L]) and ESR (>20 mm/h for men and >30 mm/h for women). We employed logistic regression for the multivariate analysis of factors associated with the pattern of discordance between CRP and ESR values. We forced variables into the equation in all the multivariate models.
Results
Serum C-Reactive Protein Concentrations in the Population
General and Demographic Data
A total of 283 (18.9%; 95% CI, 16.9–21.0%) participants had high (>0.5 mg/dL [>5 mg/L]) CRP concentrations. A total of 111 (7.4%) participants had CRP concentrations >1 mg/dL (10 mg/L). Only 12 (0.8%) participants had CRP concentrations >3 mg/dL (30 mg/L). Serum CRP concentrations significantly increased with age in the univariate analyses (Table 1). However, the positive association between age and CRP concentrations was attenuated after adjusting for covariates (Table 2). Serum CRP concentrations were not significantly different in the men and women in either the univariate (Table 1) or multivariate analyses (Table 2).
C-Reactive Protein Concentrations According to Demographics, Lifestyle Factors, and Metabolic Abnormalities*
Multivariate Analysis. Linear Regression of C-Reactive Protein (log10-Transformed) in Relation to Demographics, Lifestyle Factors, and Metabolic Factors
Lifestyle Factors
Light alcohol consumption was negatively associated with CRP concentrations, with the light drinkers having lower CRP concentrations than the abstainers in the univariate analysis (Table 1). After adjusting for covariates, light alcohol drinking was still significantly and independently associated with lower CRP concentrations, and even moderate alcohol consumption tended to be associated with lower CRP concentrations (Table 2). Serum CRP concentrations were not significantly different in the smokers or ex-smokers than in the never smokers in the univariate analysis (Table 1). However, current smoking tended to be associated with higher CRP concentrations after adjusting for covariates in the multivariate analyses (Table 2). In the univariate analyses, serum CRP concentrations were lower in the participants who engaged in moderate to high physical activity than those who engaged in low physical activity (Table 1). However, the association between physical activity and lower CRP concentrations was attenuated mainly after adjusting for covariates in the multivariate analyses (Table 2).
Metabolic Factors
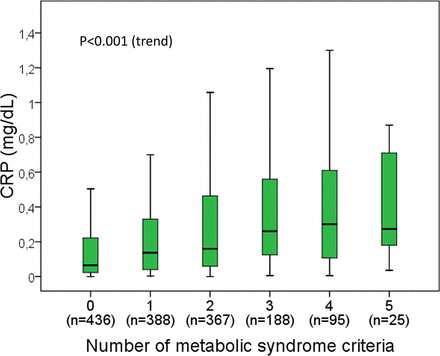
Serum CRP concentrations were significantly higher in overweight and obese individuals than those with normal weight (Table 1). Moreover, there was a significant trend of increasing serum CRP concentrations with increasing BMI category (P < .001). Median CRP concentrations were 4 times higher in the participants with obesity than in those with normal weight (Table 1). In the multivariate analyses, the positive association between BMI and CRP concentrations was still present after adjusting for age, sex, alcohol consumption, smoking, physical activity, and the presence of metabolic syndrome (Table 2). In the linear regression model, BMI explained 15.7% (R2 = 0.157) of the variation in serum CRP concentrations. The remaining covariates added only a minor explanation for the CRP variation (Table 2). Likewise, the presence of metabolic syndrome was positively associated with serum CRP concentrations in the univariate analysis (Table 1). Moreover, the participants who met any of the 5 metabolic syndrome criteria showed higher CRP concentrations than those who met none of the criteria (P < .001 in all cases, data not shown). Furthermore, serum CRP concentrations tended to increase in relation to the number of metabolic syndrome criteria per individual (Figure 1). However, the association between metabolic syndrome and CRP concentrations was attenuated by adjusting for covariates, particularly BMI (Table 2).
Boxplot of serum C-reactive protein concentrations in relation to the number of metabolic syndrome criteria. Outliers (values outside 1.5 times the interquartile range above the upper quartile and below the lower quartile) are not represented but are included in the analyses. P value was obtained with the Jonckheere-Terpstra trend test.
Correlation and Agreement between C-Reactive Protein, Erythrocyte Sedimentation Rate, and Interleukin-6 Values
The correlation between CRP concentrations and ESR values was statistically significant but not perfect (r = 0.391; n = 1472; P < .001). Most (n = 1123, 74.9%) of the participants showed both normal CRP concentrations and normal ESR values. Sixty-nine (4.6%) participants showed both high CRP concentrations and high ESR values. A total of 72 (4.8%) participants showed high ESR values and normal CRP concentrations, and 208 (13.8%) showed high CRP concentrations and normal ESR values. Table 3 presents a comparison of the demographic, lifestyle, and metabolic factors among the categories of agreement and disagreement between the CRP and ESR values.
Comparison of Demographics, Lifestyle Factors, Metabolic Abnormalities among Categories of Agreement and Discordance between C-Reactive Protein and Erythrocyte Sedimentation Rate Values
In the univariate analyses, participants with the pattern of high ESR and normal CRP showed significantly greater age, BMI, and presence of metabolic syndrome than in those from the reference category (normal CRP and normal ESR, Table 3). In the multivariate analysis (logistic regression), the pattern of high ESR and normal CRP was only significantly associated with age (Table 4). Among the 72 participants with high ESR and normal CRP, 35 (48.6%) were older than 65 years.
Multivariate Analysis (Logistic Regression) of Factors Associated with Discordance between C-Reactive Protein and Erythrocyte Sedimentation Rate*,†
In the univariate analyses, participants with the pattern of high CRP and normal ESR showed significantly greater age, a higher frequency of the female sex, higher BMI, and a greater presence of metabolic syndrome than those from the reference category (normal CRP and normal ESR), as well as lower alcohol consumption (Table 3). In the multivariate analysis (logistic regression), the pattern of high CRP and normal ESR was only significantly associated with BMI (Table 4). Among the 208 participants with high CRP and normal ESR, 174 (83.6%) had overweight (n = 69) or obesity (n = 105).
The correlation between serum IL-6 and CRP concentrations (r = 0.280; n = 1499; P < .001) was stronger than that observed between serum IL-6 and ESR values (r = 0.120; n = 1572; P < .001). Serum IL-6 concentrations were increased (>5.9 pg/mL) in 69 of the 283 (24.4%) participants with high CRP and in 76 of the 1216 (6.3%) participants with low serum CRP concentrations. Serum IL-6 concentrations were higher among the participants with high CRP and normal ESR than those with high ESR and normal CRP (P = .016, Table 3).
Discussion
The present study shows that increased CRP concentrations, with or without increased ESR occur in a sizeable proportion of adults with no apparent inflammatory disease. Determinations of serum CRP concentrations and ESR values are intended for similar purposes (diagnosis and monitoring of inflammatory diseases) but are distinct measurements influenced by different factors. Serum CRP concentrations are greatly influenced by BMI and related metabolic abnormalities, as shown in the present and previous studies,38⇓⇓–41, which suggest a state of low-grade systemic inflammation in people with overweight or obesity.40,42 Not surprisingly, increased serum CRP concentrations are a marker of cardiovascular risk.43 To a minor extent, CRP is negatively influenced by light-to-moderate alcohol consumption, in agreement with the anti-inflammatory effects of low-dose alcohol intake, as shown in this and previous studies.44 We also observed higher CRP concentrations in smokers, which is consistent with previous studies.38,41 In our experience, the association between CRP concentrations and age and regular physical exercise reported in previous studies,45,46 was attenuated mainly after adjusting for covariates. In agreement with previous reports,38,39 sex had no significant influence on serum CRP concentrations. In contrast, the ESR is highly influenced by sex (being higher in women) and aging.30 To a minor extent, the ESR is positively influenced by BMI, the presence of metabolic syndrome, and smoking, whereas it is negatively influenced by light alcohol drinking and high regular physical activity.30
The present adult-population-based study, which included individuals with no apparent inflammatory disease that could cause increased CRP or ESR, shows that the finding of the discordant pattern of high ESR and normal CRP levels (present in 4.8% of individuals) is associated with older age, whereas high CRP and normal ESR (present in 13.8% of individuals) is associated with higher BMI, which was the main determinant of serum CRP concentrations in that population. Few studies have addressed the factors associated with CRP/ESR discordances. Costenbader et al studied 2065 patients from a hospital and found that rheumatoid arthritis and low albumin levels were associated with high CRP with low ESR (present in 1.5% of cases). In contrast, infection, renal failure, and low albumin levels were associated with the pattern of high ESR and low CRP (present in 2.6% of patients).21 In that study, CRP/ESR discordance was defined by results differing by 2 tertiles.21 Colombet et al studied 5777 patients from a hospital and found that the pattern of high CRP and normal ESR was present in 5% of cases, and that the pattern of high ESR and normal CRP was present in 28% of patients.22 A detailed study of 100 discordant cases showed that the pattern of high CRP and normal ESR was associated with active inflammatory diseases, whereas high ESR and normal CRP were associated with resolving inflammatory disease,22 probably due to the slower normalization of ESR in these cases.12 Feldman et al studied 2065 patients from a hospital and found that infections, myocardial infarction, and venous thromboembolism were associated with high CRP and low ESR (present in 6% of cases), whereas connective tissue diseases and cerebrovascular diseases were associated with high ESR and low CRP (present in 6% of patients).23 In that study, CRP/ESR discordance was defined by results differing by 2 or 3 quartiles.23 Hansson et al studied 607 individuals with CRP and ESR orders from general practitioners, mixing routine controls with patients with active infection or inflammatory disease or in their convalescence phase.29 In general, active infection and inflammatory disease were associated with high CRP and normal ESR (present in 16% of patients), whereas resolving infection and an age >50 years were associated with high ESR and normal CRP (present in 5% of cases).29 Osei-Bimpong et al studied a series of 295 individuals with CRP and ESR orders from general practitioners. The patients had no significant symptoms or detectable clinical signs and had not been hospitalized for at least 6 weeks before the study. However, the authors did not investigate the causes of discordance other than age.27 To the best of our knowledge, our study is the first to comprehensively investigate the factors associated with ESR/CRP discordance in a general adult population with no overt inflammatory disease.
In general, cytokines produced during the inflammation process stimulate tissues (particularly the liver) to produce reactants (such as CRP and fibrinogen), which in turn (particularly fibrinogen) increase the ESR.3,11 In this scenario, CRP and ESR can be viewed as second-hand and third-hand markers of inflammation, respectively. Despite this and the fact that they are more than a century old, these tests are still widely employed.10 However, direct measurement of serum cytokines is gaining attention as routine, first-hand markers of inflammation. Although serum IL-6 is not a mainstream laboratory determination yet, we investigated the relationship between IL-6 and ESR, CRP, and their discordances. Serum IL-6 concentrations showed a higher correlation with serum CRP concentrations than with ESR values. Serum IL-6 concentrations were, therefore, higher among the individuals with high CRP and normal ESR than among those with high ESR and normal CRP, which is consistent with IL-6 as the main driver of CRP synthesis.3,4 Moreover, this is consistent with an increase in serum IL-6 concentrations in individuals with obesity, as observed in the present series37 and previous studies.47 Furthermore, elevated CRP and IL-6 levels predict the onset of type 2 diabetes mellitus.47 However, future studies on inflammation markers in the general population should consider that IL-6 and CRP levels are not strictly correlated. Only a quarter of individuals with high CRP also display high IL-6 concentrations.
Our study has certain limitations that should be acknowledged, the first of which is its cross-sectional design, which has inherent temporal ambiguity, limiting any inference of causality. Lifestyle factors (alcohol consumption, smoking, and physical activity) were assessed by physician-administered questionnaires. Although multivariate analyses were performed, confounders could remain a limitation in observational studies. Commercial methods were used for laboratory determinations. Of note, laboratory methods have evolved, and absolute values in this study may not correlate with those reported in previous studies. We measured wide-range CRP (wrCRP), which shows good correlation with high-sensitivity CRP (hsCRP) and may be used as a reasonable routine assay to evaluate the cardiovascular risk in patients undergoing a routine annual checkup at a lower cost.48 On the other hand, the random sampling, population basis, sample size, participation rate, and standardized methods could be considered strengths of the study. In terms of external validity, the conclusions can be applied only to similar populations of adults with no overt disease.
The results of the present study cannot serve to justify the systematic use of ESR and CRP in tandem, nor their systematic use in asymptomatic patients. However, it is a fact that the automatization of CRP and ESR measurements35,36 facilitates their widespread use in patients who are asymptomatic or mildly symptomatic and as a predictive marker (particularly CRP) of acute cardiovascular events including ischemic heart disease43 and stroke.49 Both ESR and CRP laboratory requests have increased in recent years in primary care, especially in some high-income countries.50 In many cases, the request as screening tests is not entirely justified, and the duplication (ESR and CRP) does not benefit.51 Moreover, mildly raised inflammatory markers in the context of nonspecific symptoms may be difficult to interpret and can lead to unnecessary further tests.52 Our results could help interpret laboratory results in that scenario. In otherwise healthy individuals, high ESR values and normal CRP concentrations are observed with increasing age, whereas CRP elevations and normal ESR values are observed with increasing BMI.
Notes
This article was externally peer reviewed.
Conflict of interest: The authors declare that they have no conflict of interest.
Funding: The study was supported by a grant from the Carlos III Institute of Health (Instituto de Salud Carlos III, PI16/01404 and PI16/01395), the Spanish Network for Additive Disorders (Red de Trastornos Adictivos, RD16/0017/0018, Spanish Ministry of Health) the Spanish Network for Preventive Activity and Health Promotion Research in Primary Care (Red de Actividades Preventivas y de Promocion de Salud en Atención Primaria, RD16/0007/0006), and the European Regional Development Fund (FEDER).
To see this article online, please go to: http://jabfm.org/content/34/5/974.full.
- Received for publication February 20, 2021.
- Revision received April 26, 2021.
- Accepted for publication April 27, 2021.