Abstract
Introduction: The auditory brainstem implant (ABI) is a neuroprosthetic device that provides sound sensations to individuals with profound hearing loss who are not candidates for a cochlear implant (CI) because of anatomic constraints. Herein we describe the ABI for family physicians.
Methods: PubMed was searched to identify articles relevant to the ABI, as well as articles that contain outcomes data for pediatric patients (age <18 years) who have undergone ABI surgery.
Results: The ABI was originally developed for patients with neurofibromatosis type 2 (NF2) who become deaf from bilateral vestibular schwannomas. Over the past decade, indications for an ABI have expanded to adult patients without tumors (without NF2) who cannot receive a CI and children with no cochlea or cochlear nerve. Outcomes among NF2 ABI users are modest compared to cochlear implant patients, but recent studies from Europe suggest that some non-tumor adult and pediatric ABI users achieve speech perception.
Conclusion: The ABI is a reasonable surgical option for children with profound hearing loss due to severe cochlear or cochlear nerve deformities. Continued prospective data collection from several clinical trials in the U.S. will provide greater understanding on long term outcomes that focus on speech intelligibility.
Hearing loss is the most common congenital and acquired sensory deficit among children. Moderate to severe hearing loss in children has long-term social and educational consequences, making early detection and treatment essential.1 In particular, sensorineural hearing loss (SNHL) occurs when sound is not efficiently transduced into electric potentials or when transmission of these signals to higher-order auditory centers is disrupted. The cochlear implant (CI) is the most successful neuroprosthesis for restoring SNHL and provides meaningful sound and speech perception to pediatric patients worldwide.
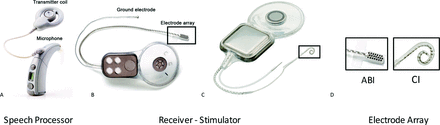
There is a small subset of pediatric patients with severe to profound SNHL loss that will not benefit from the CI due to a small or absent cochlea or auditory nerve, or scarring of the inner ear due to infection or trauma. Emerging data from the U.S. and abroad suggest that the auditory brainstem implant (ABI) can provide hearing sensations to this special cohort of deaf children who do not qualify for the CI. The CI bypasses nonfunctioning inner and outer hair cells of the cochlea to electrically stimulate the surviving first order auditory neurons called spiral ganglion cells. The ABI differs from the CI as it bypasses the damaged or absent cochlea and cochlear nerve to directly stimulate second order neurons of the auditory pathways in the brainstem called the cochlear nucleus (Figure 1).
A: Externally worn components of the ABI (speech processor), including the microphone and transmitter coil. B: Implanted components of the ABI, including the electrode array, ground electrode, and receiver-stimulator. C: Implanted components of the cochlear implant also include the electrode array, receiver stimulator, and ground receiver-stimulator. D. Comparison of ABI and CI electrode arrays. Photos provided courtesy of CochlearTM Americas, © 2009 Cochlear Americas. ABI, auditory brainstem implant; CI, cochlear implant.
History of the ABI
The ABI was first developed in the late 1970s by researchers at the House Ear Institute in Los Angeles.2 Unlike a CI, ABI electrodes bypass the cochlea and cochlear nerve to stimulate the cochlear nucleus (auditory brainstem). The original goal of the ABI was to provide sound sensations to patients with neurofibromatosis type 2 (NF2).2 Overall outcomes of adult ABI users are modest compared with those of CI users.2 The majority of ABI users with NF2 have sound awareness that aids in lip-reading and sound localization, but fewer patients have speech perception compared to CI recipients.2
Auditory Implants among the Pediatric Population
Indications for an ABI have recently expanded to children without tumors (no NF2) but with congenital deafness who are not candidates for CI2 (Table 1). Outcomes from Europe suggest that speech perception without lip-reading may be possible with an ABI in patients without NF2,3 and emerging data suggest that oral language development is possible with the ABI in some children.4 Based on these data, four separate U.S. clinical trials were initiated to determine the safety and efficacy of the ABI in the non-NF2 pediatric population. Indeed, before pediatric ABI surgery in the United States, some US-based patients without NF2 traveled to Europe to receive an ABI. A recent systematic review analyzed a total of 43 pediatric patients implanted with an ABI in 4 different studies.5 At follow-up 5 years after ABI surgery, 47.9% of pediatric ABI users without NF2 achieved a degree of speech perception.
Contraindications and Complications of an ABI
Contraindications for an ABI include severe neurodevelopmental delay, central causes of deafness, and anatomic abnormalities that may result in poor performance with an ABI4 (Table 1). ABI surgery requires a craniotomy and is associated with greater morbidity than CI surgery. One of the most common complications associated with ABI surgery is cerebrospinal fluid leakage.5 Other potential complications of auditory brainstem implantation include meningitis, cerebellar contusion and hemorrhage, facial nerve dysfunction and device failure.5
Conclusion
In summary, the ABI is reasonable surgical option for children with profound hearing loss at experienced tertiary care centers, in whom a CI would not be effective due to anatomic considerations. Because the indication for an ABI in this cohort is still new, ongoing prospective studies are necessary to determine the safety and long-term efficacy of the ABI in children.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
- Received for publication August 16, 2015.
- Revision received November 15, 2015.
- Accepted for publication November 17, 2015.