Abstract
Introduction: Academic detailing, patient-panel management, and mailed, stool-based testing have each been utilized to increase colorectal cancer (CRC) screening in rural clinics. The effectiveness of combining these interventions to increase CRC screening during COVID-19 restrictions was unclear.
Methods: We explored the effects of a multi-component intervention including academic detailing, active patient panel management, and mailed MT-sDNA testing on colorectal cancer screening in our rural family medicine clinic. Baseline interventions included EMR-based provider alerts and mailed patient reminders. Our intervention (March–May 2020) and follow-up periods (June–August 2020) coincided with the initial COVID-19 surge, giving us the opportunity to observe the effects of our intervention during COVID-19 restrictions.
Results: A total of 407 patients were eligible and overdue for colorectal cancer screening. Our clinic’s CRC screening rate increased significantly after intervention (69.7%) as compared with before (64.3%) (P = <0.01; 95%CI = 5.39-5.4). Our clinic’s CRC screening rates increased significantly during the initial 3 months of the COVID-19 surge (67.8%) compared with the same period the prior year. (62.3%) (P = .003; 95%CI = 3.4-7.6). Our CRC screening rates increased after intervention (69.7%) compared with our regional health system (67%) (P = <0.01; 95%CI = 2.6-2.77). Our weekly stool-based CRC screening increased (94% increase) compared with other health systems nationally (61 to 83% decrease).
Discussion: A multi-component intervention, including academic detailing, panel management, and mailed MT-sDNA testing, can lead to significant increases in CRC screening in a rural family medicine clinic, empowering providers to maintain an effective CRC screening outreach during COVID-19 related restrictions.
Introduction
Colorectal cancer (CRC) has the third highest cancer incidence among both men and women in the United States with 80,690 new cases in men and 70,340 new cases in women based on 2022 estimates.1 Colorectal cancer is the third most common cause of cancer mortality for males (28,400 deaths) and females (24,180 deaths) in the US based on 2022 estimates.1
Screening for colorectal cancer is recommended for asymptomatic men and women ages 50–75 (ages 45–49 grade B recommendation) at average risk via the following: colonoscopy every 10 year; flexible sigmoidoscopy every 10 years + Fecal Immunochemical Test (FIT) every year; flexible sigmoidoscopy every 5 years; CT colonography every 5 years; multitarget stool DNA test (sDNA-FIT) every 1–3 years or high-sensitivity guaiac fecal occult blood test (gFOBT) or Fecal Immunochemical Test (FIT) test annually.2
Despite multiple effective screening methods, CRC screening is often underutilized. Only 63.4% of females and 61.9% of males are up to date on CRC screening.3 In addition, state to state and rural/urban colorectal cancer screening disparities are recognized. At the state level, screening rates ranged from a high of 76.3% (Massachusetts) to a low of 58.8% (New Mexico). Nationally, screening rates for urban populations were 68.2% compared with a rural screening rate of 65.5%. States with the highest screening rates had the smallest urban-rural disparities (74.6 vs 73.0%) whereas states with the lowest screening rates had the highest urban-rural disparities (61.3% vs 56.9%).4
Interventions to increase CRC screening can be categorized as: interventions to increase provider delivery of screening services (provider assessment and feedback; provider reminders); Interventions to increase community demand (patient reminders; small media; group education) and interventions to increase community access (reducing structural barriers; reducing patient costs).5 The largest screening increases were seen among multicomponent interventions that combined approaches from each of the 3 strategies.5
One approach to increasing provider delivery is academic detailing (AD). Academic detailing is defined as an “interactive educational outreach to physicians to provide unbiased, noncommercial, evidence-based information about medications and other therapeutic decisions with the goal of improving patient care.”6 This approach parallels the “marketing” strategies used by pharmaceutical sales representative (“detailers”) to increase use of their company’s products. However, academic detailing is a peer to peer educational outreach in which medical professionals go to colleagues offices and provide brief, objective information to optimize implementation of evidence-based guidelines.6 Academic detailing involved a 6 step process: introductions; needs assessment; key message and benefits; handling objections; summary; closing the visit.6 Academic detailing can influence breast cancer screening,7 pediatric developmental screening,8 HIV testing9 and family physician prescribing behavior.10 Academic detailing can lead to increased rates of screening colonoscopies and fecal occult blood testing in rural settings.11 Academic detailing to increase CRC screening has proven both feasible and acceptable in a metropolitan12 and rural13 setting. However, although AD may be clinically effective in improving CRC screening, some have questioned its cost effectiveness.14
Patient panel management is one approach to increasing community demand and access to screening services. Panel management is defined as “a set of tools and processes for population care that are applied systematically at the level of a primary care panel, with PCP’s directing proactive care for their empaneled patients.”15 Panel management involves “identifying and reaching out to patients in the panel of a primary care practice who have unmet preventative and chronic condition care needs.”16 Active panel management is regarded as one of the key building blocks to high -performing primary care practices.17 Active panel management may be a central component of a continuous quality improvement approach to increasing CRC screening.18
One important intervention shown to increase community access to CRC screening is the mailed FIT test. Interventions using mailed FIT significantly increase CRC screening completion rates compared with usual care.19 Provision of CRC screening kits by direct mail, use of preaddressed stamped return envelopes and patient reminders seem to be effective in increasing CRC screening in rural and low income populations.20 Multi-target Stool DNA (MT-sDNA) tests combine the features of direct mail, preaddressed postage paid return packaging and patient reminders with increased sensitivity but slightly reduced specificity compare with FIT testing.21
In March 2020, the World Health Organization (WHO) designated the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak a pandemic.22 In addition in March 2020, the American College of Surgeons issued recommendations that hospitals and health systems minimize, postpone or cancel elective operations and endoscopies in an effort to minimize unnecessary SARS-CoV-2 transmissions and direct health care resources toward caring for the surge of SARS-CoV-2 patients.23 Subsequent to these and similar guidelines, issued in other nations, colorectal cancer screening declined significantly both in the United States and internationally.24
We began our intervention to increase our clinics colorectal cancer screening in March of 2020, coincidental to and concurrent with the initial SARS-CoV-2 surge. Therefore, we faced the added challenge of trying to increase CRC screening in our rural, family medicine clinic during a time when CRC screening was declining nationally and internationally.24 The purpose of this study was to examine the effects of a multicomponent intervention, centered on academic detailing, patient-panel management and mailed MT-sDNA tests, on CRC screening in a rural family medicine clinic during SARs-CoV-2 related restrictions on endoscopic procedures.
Methods
Our study was a within- groups (prepost) and between groups (intervention – control) design and was not randomized. We “controlled” our study by comparing our clinic results against: 1) our clinic’s CRC screening data from the year before the SARs-CoV-2 pandemic (March–June 2019); 2) our overall health system CRC screening rates; 3) CRC screening data from other health systems for the same time period as reported in the literature.25–27 All researchers were trained in Good Clinical Practice for the protection of human subjects and our study was approved by our Institutional Review Board (IRB).
Our population was age eligible adults in our rural, Midwest family medicine clinic. Inclusion criteria were asymptomatic male and female patients, ages 50–75, at average risk of CRC who were due/overdue for CRC screening (our protocol was established before the USPSTF 2021 grade B recommendation2 that CRC screening begin at age 45). Frequency of recommended screening depended on the method used. Exclusion criteria included: previous gastrointestinal cancer; Lynch syndrome; family adenomatous polyposis; inflammatory bowel disease; patients symptomatic on presentation (rectal bleeding) requiring diagnostic colonoscopies; patients with abnormal results on previous CRC screening necessitating more frequent colonoscopies; and patients on hospice.2
Our health system already had 2 interventions in place to increase CRC screening before our study: EMR based provider alerts of care gaps and reminders mailed to patients overdue for screening. Our baseline (preintervention) CRC screening rates are reflective of these 2 interventions already being in place.
We conducted a phone and e-mail survey among medical providers in our health system who had the highest CRC screening rates (exemplar28 providers) to identify their best practices. Most of them were skilled in active patient panel management, using the EMR to identify patients on their panel overdue for health screenings and to facilitate these screenings. Many of these exemplar providers also utilized mailed stool-based CRC screening that included addressed, postage- paid return packaging.
We conducted 2, brief online academic detailing sessions with our clinic providers in early March 2020 which included a review of: colorectal cancer screening guidelines; how to access their panel in the EMR and identify patients overdue for screening; how to order the MT-sDNA test and track patient completion and results. Providers were encouraged to dedicate 1 hour each week to panel management, during which they: identified patients overdue for screening, called and educated them about CRC screening and, if appropriate, recommend home based MT-sDNA testing. The intervention period was March through end of May 2020 and outcome data were tracked through August 2020.
Our multi-component intervention involved several strategies in accordance with the Community Preventive Service Task Force Recommendations5 and is summarized in Table 1.
Multi-Component CRC Screening Intervention in Rural Family Medicine Clinic March–May 2020
Our independent (predictor) variable was our multi-component CRC screening intervention. Our dependent (outcome) variables included: Stool-Based CRC screenings ordered per month; Stool-Based CRC screenings completed per month; Stool- based CRC screenings ordered or completed per week; and overall colorectal cancer screening rates. The colorectal cancer screening rate statistic was based on the number of eligible patients who completed screening/number of patients eligible for screening during the time period. Stool based CRC screenings ordered and completed per month were compared before (Feb/March) and after (April/May) intervention. Stool based CRC screenings ordered/completed per week were compared within-groups (before and after) for our clinic and between groups (our clinic results compared with results from other clinics cited in the literature from the same time period25–27). Change in overall CRC screening rates are compared with-in groups (pre and post intervention), between our clinic (intervention) and our regional health system (control) and between our clinic during the initial 4 months (March–June, 2020) of the SARS-CoV-2 surge and our clinics data during the same time period the year prior.
Within-group (before and after) analysis was performed using paired sample t test. Between group analysis was performed by comparing our study outcomes with regional and national test values cited in the literature25–27 using 1 -sample t test. Statistical analysis was conducted with the Statistical Package for Social Sciences (SPSS) version 25.
Results
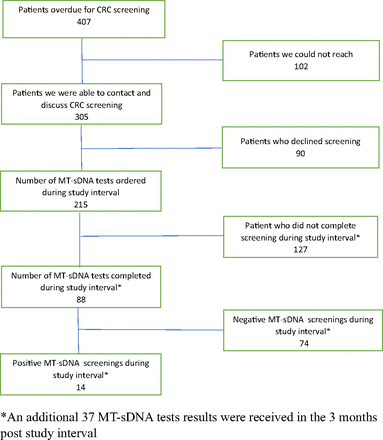
Our study population consisted of 407 patients in our rural Midwest family medicine clinic who were eligible and overdue for colorectal cancer screening. Patient eligibility, participation and disposition is illustrated in Figure 1.
Flow diagram for patient eligibility and participation in Multi-component colorectal cancer (CRC) screening study March–May, 2020.
Patient demographics were obtained via a self- report survey. Overall, our study population tended to be older, white, middle class, female, and more likely to report higher education and having a primary care provider compared with county wide, U.S Census Bureau statistics. The older age of our sample is likely a result of age criteria to be eligible for CRC screening. The higher rate of having a PCP may be due to the fact our sample population was drawn from our electronic medical record. The gender, educational and socio-economic differences in of our sample may be reflective of self- sampling bias and/or differences between our definition of terms and that of the U.S Census Bureau.29 Patient demographic of our sample and for the county wide population are shown in Table 2.
Demographic Characteristics of Multi-Component Study Sample and County-Wide Population March 2020
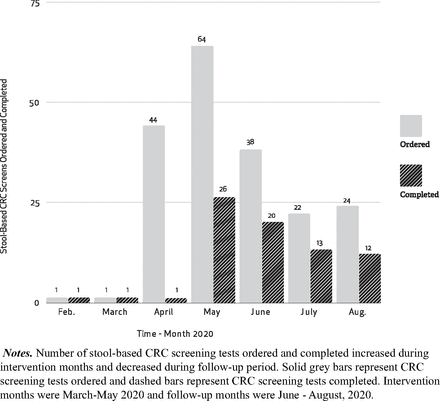
We observed a significant increase in the number of stool- based CRC screenings ordered per month in our clinic after intervention (n = 64) as compared with before (n = 1) (t = 4.49; P = <0.01; 95% CI = 1.96-5.46) We observed a significant increase in the number of stool based CRC screening completed per month in our clinic after intervention (n = 26) as compared with before (n = 1) (t = 2.71; P = .02; 95% CI = 0.32-2.62). (See Figure 2).
Stool-based CRC screening ordered and completed by month 2020 in a rural family medicine clinic.
We observed a significant increase in weekly stool-based CRC screenings in our clinic after intervention (average 16 screens per week) as compared with before (average 1 screen per week) (t = 10.94; P = <0.01; 95% CI = 0.71-1.05). We observed a significant increase in our clinics weekly stool – based CRC screening (average of 1 screen per week to 16 per week) when compared with our regional health system25 (average of 210 per week to 51 per week) (t = 2719; P = <0.01; 95% CI = 159.8 to 160.1). We observed a significant increase in our clinic’s average weekly stool-based CRC screening when compared with health systems in the San Francisco26 (370 per week to 60 per week) (t = 5286; P = <0.01; 95% CI = 310.8 to 311) and Los Angeles areas27 (154 per week to 60 per week) (t = 1614; P = <0.01; 95%CI = 94.8 to 9). Although the total weekly stool-base CRC screening of these larger health systems was still higher than our individual clinic, our stool-based CRC screens were trending up during COVID- 19 restrictions (94% increase) whereas our regional and the national health system rates were trending down (61 to 83% decrease) during the same time period. See Table 3.
Clinic Stool-Based CRC Screening Orders per Week Compared with Other Health Systems Pre and Post Initial COVID-19 Surge, 2020
We observed a significant increase in the overall CRC screening rate in our clinic after intervention (69.7%) as compared with before intervention. (64.3%) (t = 1231.9; P = <0.01; 95% CI = 5.39-5.4;). We observed a significant increase in the overall CRC screening rates in our clinic during the initial 4 months (March–June 2020) of the SARS-CoV-2 surge (67.8%) compared with our clinic CRC screening rate from the same period the year prior (62.3%) (t = 8.5; P = .003; 95% CI = 3.4 to 7.6). Our health system experience a significant decrease in overall CRC screening rates during the initial 4 months of the SARS-CoV-2 surge (68.3%) compared with the same 4 month time period the year prior (74.5%) (t = 7.3; P = .005; 95% CI = 3.5–8.7) We observed a significant increase in our overall clinic CRC screening rates (69.7%) as compared with our regional health system rates (67%) (t = 74.2; P = <0.01; 95% CI = 2.6-2.77). See Figure 3.
Colorectal cancer screening for clinic and health system by month 2019 and 2020.
During our 3-month postintervention follow up (June–August 2020) we observed a decrease in some clinic outcome measures including: stool-based CRC screens orders (38, 22, and 24 orders placed) and stool-based CRC screens completed (20, 13, and 12 completed). After our intervention period, our clinicians focused their academic detailing and panel management efforts onto other preventive services (breast cancer screening). It is possible that our decline in CRC screening during the follow up phase was due to this abrupt redirection of our preventive screening efforts.
Discussion
Our multi-component intervention, centered on academic detailing, patient-panel management and mailed MT-sDNA, led to a significant increase in CRC screening in our rural family medicine clinic. Of additional importance, we were able to achieve this significant increase during a time when CRC screening was declining regionally,25 nationally,26,27 and internationally24 due to SARS-CoV-2 restrictions. Our study parallels and furthers the evidence that increased use of mailed stool -based CRC screening may counterbalance and offset SARS-CoV-2 induced decreases in screening endoscopies.30
Our findings confirm and extend the evidence a that multi-component intervention, focused on increasing provider delivery, community demand and community access, can significantly increase CRC screening.5 The individual and separate effects of academic detailing11 and mailed FIT19,20 on CRC screening have been studied previously. However, the literature on the effects of patient-panel management on CRC screening was limited.18 Our study demonstrates that active panel management can be an important component, when combined with other strategies, to increased colorectal screening. To our knowledge, ours is the first study to demonstrate the effectiveness of combining academic detailing, panel management and mailed MT-sDNA testing during SARS-Cov-2 restrictions.
Our interventions are applicable to busy primary care providers and under-resourced patients in medically underserved settings. Academic detailers come to the clinic, eliminating the time and expense of CME travel. Medical assistants can be trained to facilitate panel management. Mailed MT-sDNA tests reduce the financial, scheduling, and transportation barriers that colonoscopies pose for patients. Other strengths and limitations of our study are detailed in Table 4.
Strengths and Limitations of Our Multi-Component Intervention to Increase CRC Screening Rates in a Rural Family Medicine Clinic During Initial COVID-19 Surge, 2020
Future studies could recruit multiple clinics from a broader geographic area and incorporate stratified sampling, so the sample was more representative of the US population distribution. A design which randomized clinics into intervention and “treatment as usual” (control) clinics would reduce the effects of confounding variables. Future studies could be designed so that the unique contributions of academic detailing, panel management and mailed MT-sDNA could be individually analyzed via multiple regression.
Conclusion
Our study demonstrates that a multi-component intervention, centered on academic detailing, active patient-panel management and mailed MT-sDNA can lead to significant increases in CRC screening in a rural family medicine clinic even during SARS-CoV-2 induced restrictions on endoscopic screening.
Acknowledgments
We would like to thank James Lyons, MD, for his contributions to research design, patient recruitment, and panel management. We would also like to thank Emily Greeson, PhD, Kathleen Lowenstein, PhD, and Michael O’Rourke, PhD, for their contribution to research design, patient recruitment, data analysis, and the patient demographic survey.
Notes
This article was externally peer reviewed.
This is the Ahead of Print version of the article.
Funding: None.
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/00/00/000.full.
- Received for publication March 1, 2023.
- Revision received July 3, 2023.
- Accepted for publication July 10, 2023.