Abstract
Introduction: When implementing interventions in primary care, tailoring implementation strategies to practice barriers can be effective, but additional work is needed to understand how to best select these strategies. This study sought to identify clinicians’ contributions to the process of tailoring implementation strategies to barriers in clinical settings.
Methods: We conducted a modified nominal group exercise involving 8 implementation scientists and 26 primary care clinicians in the WWAMI region Practice and Research Network. Each group identified implementation strategies it felt would best address barriers to using a cardiovascular disease (CVD) risk calculator previously identified across 44 primary care clinics from the Healthy Hearts Northwest pragmatic trial (2015 to 2018). These barriers had been mapped beforehand to the Consolidated Framework for Implementation Research (CFIR) domains. We examined similarities and differences in the strategies that 30% or more of each group identified (agreed-on strategies) for each barrier and for barriers in each CFIR domain. We used the results to demonstrate how strategies might be tailored to individual clinics.
Results: Clinicians selected 23 implementation strategies to address 1 or more of the 13 barriers; implementation scientists selected 35. The 2 groups agreed on at least 1 strategy for barriers in each CFIR domain: Inner Setting, Outer Setting, Intervention Characteristics, Characteristics of Individuals, and Process. Conducting local needs assessment and assessing for readiness/identifying barriers and facilitators were the 2 most common implementation strategies chosen only by clinicians.
Conclusions: Clinician stakeholders identified implementation strategies that augmented those chosen by implementation scientists, suggesting that codesign of implementation processes between implementation scientists and clinicians may strengthen the process of tailoring strategies to overcome implementation barriers.
- Cardiology
- Cardiovascular Diseases
- Decision Support Tools
- Implementation Science
- Needs Assessment
- Primary Health Care
- Quality Improvement
- Stakeholder Participation
Introduction
Accelerating the adoption of evidence-based interventions (EBIs) into practice is critical to improving individual and population health. Over the past 2 decades, there has been limited implementation of health interventions1⇓–3 critical to improving population health. Cardiovascular disease (CVD), responsible for the greatest morbidity and mortality across the US,4 fits the profile of having multiple EBIs that support its prevention yet are inadequately implemented.5,6 The AHA’s Million Hearts Initiative has worked since 2012 to support implementation of critical EBIs that could substantially decrease CVD morbidity and mortality – tobacco control, hypertension detection and management, cholesterol management and aspirin (ABCS).7 However, this longstanding national initiative has not met its ABCS targets, with only about half of those with hypertension having controlled blood pressure and just over half (54.5%) of those who qualify for statins using them.8 Effective implementation of EBIs such as these within clinical practice requires a level of quality improvement capacity that to this point has been out of reach of many smaller primary care clinics.9,10
The Agency for Health care Research and Quality addressed this challenge head-on with its EvidenceNow initiative, which funded 7 cooperatives nationally to test different strategies to support small- and medium-sized primary care clinics in integrating evidence-based approaches to reduce their patients’ CVD risk.11 Healthy Hearts Northwest (H2N), 1 of the 7 cooperatives, sought to increase use of a CVD risk calculator to inform recommendations for lifestyle changes and/or cholesterol lowering medications (eg, statins) for prevention of CVD using an evidence-based implementation strategy, virtual educational outreach visits with prescribers and staff.12,13 A CVD risk calculator uses commonly available demographic and clinical information (eg, age, sex, total and HDL cholesterol) to estimate an individual’s risk of cardiovascular disease over a specified time period, typically 10 years. Health care providers can use a CVD risk calculator as a tool to determine whether interventions such as statins might help prevent cardiovascular disease for an individual in their practice. Using notes taken during the educational outreach visits, the H2N study team identified barriers to the use of a CVD risk calculator.14 These barriers were present at multiple levels—clinic, provider, patient, and technology—suggesting that implementation strategies beyond educational outreach may be needed to effect practice change.
Implementation strategies are methods or techniques used to enhance the adoption, implementation, and sustainability of a clinical program or practice.15 Tailoring implementation strategies, defined as selecting those strategies or a combination of strategies to address the unique needs of or barriers to the implementation efforts, has received increasing attention in the literature,16⇓⇓⇓–20 including 2 Cochrane reviews.21,22 The latest Cochrane review concluded that tailored strategies can be effective, but that additional work is needed to understand how to best select tailored strategies for implementing interventions.
Several approaches for tailoring implementation strategies have been proposed,17,23 such as Intervention Mapping,24 concept mapping,25 conjoint analysis,26 and system dynamics modeling.27 Although most of these approaches incorporate some stakeholder inputs, they usually involve a complex and iterative implementation scientist-driven process of tailoring and selecting implementation strategies that may not be feasible or practical for implementing evidence into primary care. We were interested in identifying the unique contribution that clinicians might add to the process of selecting implementation strategies to address specific barriers in primary care settings. To accomplish this, we used barriers previously identified during the H2N educational outreach visits14 to integrate the expertise of both clinicians and implementation scientists in the development of a more pragmatic tailored approach to implementing use of a CVD risk calculator in primary care clinics. Through this work, we hoped to help close the divide between the science and the practice of implementation in real-world primary care settings and bring clinicians’ perspectives to tailoring implementation of interventions into practice.
Methods
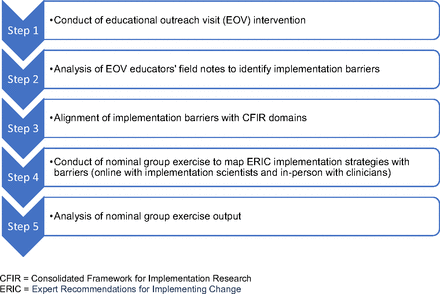
Our research team conducted a modified nominal group exercise involving a group of implementation scientists and a group of primary care clinicians. Each group identified the implementation strategies that it felt would best address the previously identified barriers that H2N clinics reported to using a CVD risk calculator, then synthesized the exercise results (Figure 1).
Overview of methods supporting this study.
Setting
The Healthy Hearts Northwest (H2N) pragmatic trial was conducted between 2015 and 2018 across 209 primary care clinics with 10 or fewer full-time equivalent family medicine or general internal medicine providers in urban and rural settings across Washington, Oregon, and Idaho. The study protocol and findings have been previously published.12,28 Briefly, the study tested different combinations of implementation strategies to increase quality improvement capacity and practice performance on the 4 ABCS of heart disease prevention: Aspirin use by high-risk individuals, Blood pressure control, Cholesterol management, and Smoking cessation. A 2-by-2 factorial design was used to compare the effectiveness of adding educational outreach visits and/or shared learning opportunities to practice facilitation. Clinics (104) were randomized to the educational outreach intervention focused on promoting routine use of a CVD risk calculator; 44 clinics participated.13 The majority of participating clinics were located in urban areas (61.4%), were family medicine specialty-focused (84.1%), and were either independently owned (38.6%) or owned by health or hospital systems (43.2%). Trained physicians conducted 30-minute interactive webinars or telephone calls with clinical care teams (eg, clinicians, pharmacists, medical assistants) within these 44 participating primary care clinics. The interaction focused on eliciting current practices and attitudes toward CVD risk calculation, as well as actual or anticipated barriers to using a CVD risk calculator, and on identifying specific strategies to overcome those barriers. After conducting a visit, the physician recorded field notes describing each clinic’s experiences with CVD risk calculation, including factors that participants reported as influencing their ability to use a CVD risk calculator.
Identification of Barriers and Matching Barriers to CFIR Domains
The H2N study team previously reported the barriers to CVD risk calculator use, then aligned these barriers with 1 or more Consolidated Framework for Implementation Research (CFIR) domains.14 The CFIR includes multiple constructs within 5 domains (Inner Setting, Outer Setting, Intervention Characteristics, Characteristics of Individuals, and Process), and can be used to describe barriers and facilitators while preparing for implementation of an evidence-based intervention.29 Briefly, 2 study team members (LT, EH) analyzed the field notes from the visits and used an inductive open coding approach in which codes describing barriers to CVD risk calculator use emerged from the data. The team identified 13 different types of implementation barriers reported by the clinical teams (eg, time constraints for clinicians/staff, concerns about variation in results from different risk calculators, patient inability or willingness to pay for statin medications, lack of buy-in by staff or providers, lack of trust in guidelines). All 5 CFIR domains were represented by the 13 implementation barriers.
Modified Nominal Group Exercise
We invited the 8 implementation scientists who published the Expert Recommendations for Implementing Change (ERIC) study that compiled and refined 73 implementation strategies to participate in an online exercise to match implementation strategies to the H2N study’s 13 identified barriers; 6 participated. The twenty-six primary care clinicians (largely family physicians with a few pharmacists and behavioral health professionals) attending the WWAMI region Practice and Research Network’s (WPRN’s) annual conference in spring 2018 participated in a 45-minute, in-person exercise similar to the online exercise. The WPRN is a primary care practice-based research network with clinical organizations in Washington, Wyoming, Alaska, Montana, and Idaho (WWAMI). These clinicians represent clinical organizations that are members of the WPRN, and are knowledgeable about their organizations’ clinics, their operations, and the care that they offer. The clinicians were not required to be familiar with the ERIC implementation strategies. Neither the implementation scientists nor the primary care clinicians were involved in the H2N study. Their input was based on their general experience of implementation science and clinical practice.
We provided both the implementation scientist and the primary care clinician participants a list of the 13 previously identified barriers to using a CVD risk calculator and the barrier definitions,14 as well as a list of the 73 implementation strategies with their published definitions from the ERIC project.30 We asked each person to identify the top 5 implementation strategies that they felt would be most effective in addressing or overcoming the identified barriers to using a CVD risk calculator. Implementation strategies could be selected more than once across the 13 implementation barriers.
Analysis
Based on the individual group results, our research team compiled a matrix that mapped strategies that the implementation scientists and clinicians chose for individual barriers. In the matrix we indicated when 2 or more of the 6 implementation science experts (30%+) and the same proportion of the 26 primary care clinicians (30%+) matched a strategy to each of the 13 barriers. We also identified whether there were agreed-on strategies that 30% or more of both implementation science experts and clinicians chose for each barrier. Based on the previously published mapping of the barriers to CFIR domains (Inner Setting, Outer Setting, Intervention Characteristics, Characteristics of Individuals, and Process), we compared which implementation strategies were chosen to address CFIR-based barrier categories by both implementation science experts and clinicians, by implementation science experts only, and by clinicians only. Next we listed the combinations of barrier types identified by the 42 H2N clinics reporting barriers. Using these barrier combinations, we drew from the agreed-on implementation strategies chosen by both groups to develop examples of how strategies could be tailored to individual clinics based on the CFIR domains where barriers exist within each clinic.
Results
Overall, clinicians and implementation scientists identified 39 of the 73 ERIC strategies as agreed-on strategies that would be most effective in addressing 1 or more of the 13 barriers to implementing the CVD risk calculator. Implementation scientists identified more agreed-on strategies (35) than clinicians (23). There was agreement between clinicians and implementation scientists on at least 1 strategy that they thought would be effective for 10 of the 13 barriers (Table 1). The 3 barriers where there was no agreement on a strategy were time constraints to using the calculator, a perception that the clinic had a limited population at risk for CVD, and thus did not justify using the calculator, and concerns that different calculators gave different results.
Agreed-Upon ERIC Implementation Strategies for Individual Barriers to Implementing the Cardiovascular Disease Risk Calculator
Clinicians and implementation scientists agreed on at least 1 strategy for all 5 of the CFIR domains represented by these barriers (Table 2). For barriers represented by the CFIR Inner Setting domain, clinicians and implementation scientists agreed on 13 implementation strategies. For barriers represented by the Outer Setting and Intervention Characteristics domains, clinicians and implementation scientists agreed on 4 implementation strategies each. Clinicians and implementation scientists agreed on 3 implementation strategies for the Process domain, and only 2 strategies for the Characteristics of Individuals domain.
Agreed-Upon ERIC Implementation Strategies for Barriers to Implementation by 5 CFIR Domains
A number of strategies were chosen only by implementation scientists, the most common being facilitation (Table 2). Four strategies were chosen by clinicians but not implementation scientists: conduct local needs assessment, assess for readiness and identify barriers and facilitators, make training dynamic, and fund and contract for clinical innovation.
There were 15 different combinations of CFIR-based barrier types (Table 3) across the 42 clinics that reported at least 1 barrier. Inner setting barriers were the most common, followed by barriers related to the Intervention Characteristics. Four of the clinics had barriers in all 5 CFIR domains; 10 clinics had barriers in 4 of the domains; and 13 clinics had barriers across 3 domains.
Number of Clinics Reporting Different Types of Barriers Categorized into 5 CFIR Domains
Figure 2 uses the results of the nominal group exercise to hypothetically show how an example implementation plan might be tailored to combinations of barriers in 2 different clinics and how the strategies chosen might change if clinicians’ input was incorporated before implementation. Practice 1 had both Inner Setting and Outer Setting barriers to use of the risk calculator. There were several strategies that both clinicians and implementation scientists agreed could help overcome these barrier types: develop a formal implementation blueprint and obtain and use patient and family feedback. Clinicians recommended the following additional strategies that an implementation team might want to consider: assess for readiness and identify barriers and facilitators (Inner Setting) and conduct educational meetings (Outer Setting). Practice 2 had barriers in 3 CFIR domains: Inner Setting, Intervention Characteristics, and Characteristics of Individuals. There were 2 strategies agreed on by both clinicians and implementation scientists to address barriers across these 3 domains: identify and prepare champions and conduct educational meetings. Additional strategies identified by implementation scientists or clinicians could be used to tailor an approach to a clinic with these barrier types. Across both clinics there were common strategies that might be considered as “core” or “foundational,” for example, facilitation and assess for readiness and identify barriers and facilitators. These foundational strategies could be augmented with other strategies tailored to the needs of a particular clinic and its barriers.
Example implementation plans tailored to clinics with different combinations of barriers. Abbreviations: EHR, Electronic Health Record; FQHC, Federally Qualified Health Center.
Discussion
Tailoring implementation strategies to context, including barriers, is recognized as critical to implementation success, but a clear understanding of how to tailor effectively is lacking.20,31,32 Current methods have largely engaged implementation scientists in the tailoring process.17,23,33 In this study, we sought to understand how clinicians might contribute to the process of tailoring implementation strategies. We used data from an assessment of implementation barriers to CVD risk calculation reported by primary care clinics to engage both clinicians and implementation scientists in a pragmatic exercise to identify the implementation strategies they believed would best overcome these barriers.
Although the results demonstrated some agreement in the implementation strategies chosen by both clinicians and implementation scientists, there were notable differences in the 2 groups’ choices. Overall, as a group the implementation scientists agreed on a greater number of strategies than the clinicians. We engaged implementation scientists responsible for developing the ERIC typology of implementation strategies. Their high level of familiarity with the strategies and the role of these strategies in implementing interventions may partially explain why they were able to identify more agreed-on strategies than the clinicians. Alternately, they may have chosen a broader range of strategies because they were less familiar with the context of primary care. Implementation scientists chose facilitation as an agreed-on strategy for 9 of the 13 barriers, whereas the clinicians never chose facilitation as a strategy. Clinicians may have been unfamiliar with this strategy. Alternately, clinicians may not have had a favorable view of facilitation based on their experience with this strategy or based on the ERIC definition of this strategy as “a process of interactive problem-solving and support that occurs within the context of a recognized need for improvement.” They may have viewed this process as requiring a significant commitment of time, compared with other strategies, or they may not have understood the ERIC definition. Given the widespread use of facilitation in implementation efforts, further work to explore this is needed.
These results also suggest that partnering with individuals from the intended implementation environments in the tailoring process can illuminate potentially important strategies that implementation scientists may not identify. In this study, clinicians chose 2 strategies that address local context that were never chosen by the implementation scientists: (1) assessing for readiness/identifying barriers and facilitators and (2) conducting a local needs assessment. In choosing the strategy “assessing for readiness/identifying barriers and facilitators,” clinicians may be sharing that understanding a clinic’s readiness for change is essential to an implementation plan’s success. Importantly, the strategy “conducting a local needs assessment” can help identify factors such as the social needs of a clinical population that may lead to unequal provision or uptake of an evidence-based intervention, in turn influencing the way in which implementation strategies can be tailored to help address existing health inequities or prevent improvement efforts from exacerbating them.34,35 There is a robust literature reporting how assessment of context is critical to tailoring, and therefore is clearly important for the success of any implementation effort.36 This finding illustrates the value of engaging stakeholders, in this case primary care clinicians, in the development of a research project’s intervention implementation strategy. Indeed, pragmatic research from the National Institutes of Health Collaboratory has shown that embedding research in care settings where researchers and clinicians collaborate enables clinicians to influence the relevancy of pragmatic research questions, improve the feasibility of the intervention, and increase clinician buy-in to conducting evidence-generating research in the clinic setting.37 A next research question is whether implementation scientists and individuals from the intervention setting (eg, clinicians, patients) together can more effectively tailor implementation strategies to improve intervention adoption and associated patient outcomes.
When we applied the findings of our exercise to 2 example clinics from the H2N study, we demonstrated that it is possible to develop tailored approaches to implementing the CVD risk calculator in these settings. Some strategies were commonly chosen by the clinician and/or implementation scientist groups for most of the barriers. These might be used as “core” implementation strategies across all clinics (eg, facilitation, identifying and preparing champions). An implementation effort might then best be served with a toolbox of additional strategies that could be applied to address specific barriers in individual clinical sites. In addition to a more informed approach to overcoming specific barriers, discussing this toolbox of additional strategies might create more engagement and buy-in from clinicians and others in the clinical settings as well as anticipate potential challenges or barriers that might inform the strategies selected for the implementation process. This process of engagement is consistent with a defined ERIC implementation strategy, “…engage a formal group of multiple kinds of stakeholders to provide input and advice on implementation efforts and to elicit recommendations for improvements,”30 which can provide a structure for guiding the choice of implementation strategies most suited to a specific clinical setting. An end result might be a practical guide for the process of choosing core implementation strategies, then conducting stakeholder-engaged tailoring of additional implementation strategies to address the barriers, facilitators and context of specific clinical environments. This guide could be useful to implementation scientists as well as practice facilitators and others in clinical settings seeking to implement evidence-based interventions.
This study’s limitations include its reporting only on a single pragmatic exercise related to a specific clinic-based intervention, CVD risk calculation, in primary care practice. Before generalizing these findings, it will be important to determine whether clinical stakeholders in a variety of settings and for multiple evidence-based interventions consistently identify unique strategies to overcome implementation barriers when compared with those chosen by implementation scientists. This study was not designed to examine how inclusion of clinician-identified strategies in an implementation plan influences the implementation process and outcomes, as well as the intervention outcomes. Further evidence is needed to determine whether this collaborative implementation planning process is important to improving clinical outcomes. When repeating this modified nominal group exercise in research focused on other EBIs, it will be important to define implementation strategies with common language that both implementation scientists and clinicians can understand, so that the 2 groups choose strategies consistently. Common language definitions might also increase the usability of this comprehensive set of strategies by clinicians and other nonimplementation scientists as they plan for implementing evidence-based interventions or participate in implementation research. Finally, in future exercises such as the 1 reported here, it will be important to provide a detailed explanation about each of the Inner Setting and clinical barriers to ensure that both implementation scientists and clinicians have a shared understanding of these challenges.
Tailoring implementation strategies to barriers when developing an implementation plan is an imprecise activity and depends on a clear understanding of the strengths of different implementation strategies and the context of the clinical setting to which an intervention is targeted. This study suggests that codesign of implementation processes between implementation scientists and clinicians in the targeted clinical environment may strengthen the process of selecting the most appropriate implementation strategies. Further research is needed to determine whether including both implementation scientists’ and clinicians’ choices of implementation strategies improves implementation outcomes and intervention effectiveness.
Acknowledgments
The authors thank the implementation scientists and clinicians in the WWAMI region Practice and Research Network (WPRN) for participating in the group exercise that serves as the basis for this work. We also thank Gina Keppel, MPH and Brenda Mollis, MA, MPH, MPA for their assistance in organizing and conducting the group exercise with clinicians at the WPRN annual conference, Seattle, Washington; March 7, 2018.
Notes
This article was externally peer reviewed.
This is the Ahead of Print version of the article.
Funding: This project was supported by Grant R18HS023908 from the Agency for Healthcare Research and Quality. Additional support was provided by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award UL1TR002319. The content is solely the responsibility of the authors and does not necessarily represent the official views of either the National Institutes of Health or the Agency for Healthcare Research and Quality.
Conflicts of interest: None.
To see this article online, please go to: http://jabfm.org/content/35/6/000.full.
- Received for publication November 9, 2021.
- Revision received February 19, 2022.
- Accepted for publication February 24, 2022.