Abstract
Purpose: Describe primary care providers’ (PCPs) barriers and facilitators to implementation of lung cancer screening programs in rural settings.
Methods: We conducted qualitative interviews with PCPs practicing in rural Oregon from November 2019 to September 2020. The interview questions and analytic framework were informed by the 2009 Consolidated Framework for Implementation Research. We used inductive and deductive approaches for analysis.
Results: We interviewed 15 key participants from 12 distinct health care systems. We identified several Consolidated Framework for Implementation Research factors affecting lung cancer screening implementation. 1) Most PCPs did not have workflows to assist in discussing screening and relied on their memory and knowledge of the patient’s history to prompt discussions. PCPs supported screening and managed the patient throughout the process. 2) PCPs reported several patient-level barriers, including geographic access to lung cancer screening scans and out-of-pocket cost concerns. 3) PCPs reported that champions are necessary to create opportunities for local practices to adopt lung cancer screening programs.
Conclusions: Rural-practicing PCPs were supportive of lung cancer screening, however workflow processes, time challenges, and patient-reported barriers remain impediments to improved screening in their clinics. We identified several areas for improvement in lung cancer screening implementation in rural primary care practices, ranging from designing clinic workflows and processes to designating clinic staff to support referral, screening, and follow-up care for patients.
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States—surpassing breast, prostate, and colon cancer-related deaths combined.1,2 Lung cancer screening using annual low-dose computed tomography (low-dose CT) increases early detection of lung cancer and reduces lung cancer mortality.3,4 Many organizations, including the United States Preventive Services Task Force, recommend lung cancer screening for high-risk individuals with some stipulations.3,5,7 For instance, patients should engage in a shared decision making interaction with a qualified clinician before making a decision on undergoing lung cancer screening.3,7,8 Shared decision making should involve high-quality communication that takes into account individual characteristics and values, as well as the amount of control the patient desires when making the actual decision.9⇓–11
Despite recommendations, lung cancer screening uptake has been slow overall, with multiple studies suggesting uneven adherence among rural patients to recommended follow-up guidelines.12⇓⇓⇓–16 Unfortunately, of all lung cancer screening eligible patients, only approximately 5 to 15% undergo screening.12,17 Rural patients are more likely than urban patients to smoke, have lung cancer, late stage lung cancer, and die from lung cancer once diagnosed.14,18,19 Yet, access to screening is more limited in rural areas compared with urban areas.15,20 For example, in 2019, only 51% (17/29) of surveyed (29/37) rural hospital-based radiology facilities offered LCS using low-dose CT in Oregon.21 Furthermore, challenges exist to implementing lung cancer as well as other screening programs (eg, diabetic eye and colon) in rural settings, such as overburdening of small rural health-care workforces, limited EHR functionality, and geographic isolation, making it important to identify system-level processes to support screening in rural settings.22⇓⇓⇓–26
Primary care providers (PCPs) often identify eligible patients, engage in shared decision making interactions, refer patients, communicate results, ensure patients are adherent to follow-up recommendations, and coordinate with diagnostic and treatment specialists should lung cancer be suspected.27 Understanding PCPs’ experience with lung cancer screening is necessary to create solutions for implementing high-quality programs in rural areas because we are currently do not know of rural-specific barriers to implementation from the PCP perspective. The purpose of this study was to describe primary care providers’ barriers and facilitators to implementation of lung cancer screening programs in rural settings. We focused on actionable mechanisms to change screening uptake and adherence.
Methods
We conducted qualitative interviews with PCPs practicing in rural Oregon between November 2019 and September 2020. Study activities were conducted in partnership with the Oregon Rural Practice-based Research Network, a network that conducts research and quality improvement projects with clinics across the state.28 Our study was approved by the VA Portland Health Care System/Oregon Health & Science University IRB (#18865). Of note, the term ‘lung cancer screening’ or ‘screening’ herein refers to the process of screening while ‘low-dose CT’ refers to the scan. We make the distinction since perceptions about the process may differ from those about the scan.
Participants and Data
We sampled participants from clinics in rural Oregon who indicated interest in participating. We recruited by snowball sampling through advertising via the Research Network newsletter (641 recipients) for 3 months and contacts (eg, practice facilitator networks, existing clinic relationships, etc.) (recipients unknown). We emphasized variation in the clinic’s affiliation and hospital designation (critical access hospitals or not). We define rural as geographic areas > 10 miles from a population center of more than 40,000, in alignment with Rural-Urban Commuting Area definitions.29,30 We included PCPs in more urban geographic areas if they self-identified as serving rural-residing patients.
A pulmonologist and health services researcher with experience in qualitative methods, conducted all interviews by phone using a semistructured interview guide (Appendix). Another investigator accompanied to take notes. We obtained consent by phone from participants before each interview. We asked about PCP perceptions of lung cancer screening, workflow processes, shared decision making, champions, and patient barriers. Herein we define workflow processes as “a series of tasks performed by various people within and between work environments to deliver care.”31 We based interview questions on the 2009 Consolidated Framework for Implementation Research, which identified factors associated with implementation; it is a theory-based guide for assessing barriers and facilitators of strategies to improve future implementation and maintenance.32 The interview guide focused on the following Framework constructs, or factors, based on previous studies and that aligned with study goals: individuals involved (eg, knowledge, beliefs, and perceptions about screening), inner setting (eg, implementation climate, structural characteristics, workflow factors, shared decision making), outer setting (eg, patient needs, resources, and barriers), and implementation process (eg, champions) (Table 1).32 The research team stopped interviews after information power on our focused topic was satisfactory.33,34
Key Construct Definitions
Interviews lasted an average of 44 minutes. Each interview was transcribed verbatim, deidentified, and verified for accuracy before analysis by team member. We performed member checking with all participants. All participants received $100 renumeration for their time and effort.
Analysis
We used Atlas.ti version 8 to organize qualitative data. We used conventional content analysis to identify and systematically code text and develop an organizational structure for coded data to help look for patterns within and across participants.35,36 First, our multidisciplinary team created a codebook using inductive and deductive approaches, including components of the interview guide. Reviewers met frequently as a group to discuss and refine the codebook. The remaining transcripts were independently coded. We met frequently to discuss findings, refine codes, and recode as needed. We resolved differences through consensus and used an audit trail and memos to ensure consistency and rigor by tracking decisions related to coding and analysis.
Results
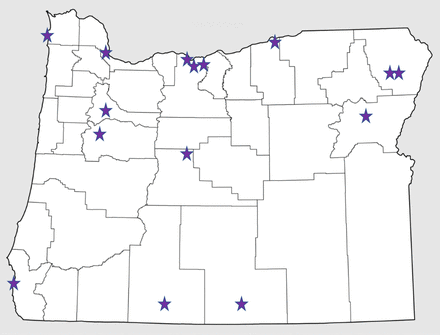
We interviewed 15 key participants who were associated with 12 distinct health care systems in Oregon (Figure 1). Informant characteristics are included in Tables 2 and 3. We identified the following constructs as affecting lung cancer screening implementation: 1) Inner setting, 2) Outer setting), and 3) Implementation process. Each construct is similar to a theme, wherein they are factors mentioned across participants that characterize experiences. While we queried about individuals involved, it did not surface as a theme in our data. There were no differences based on training level (ie, physician vs advanced practice practitioner), presence of a program, or distance to the low-dose CT.
Map of key participant locations.
Self-Reported Participant and Institution Characteristics
Aggregate Self-Reported Informant and Institution Characteristics
Lung Cancer Screening Workflow Process Improvement Suggestions
CFIR Domain: Inner Setting
Construct: Implementation Climate
The majority of participants noted the importance and value of lung cancer screening implementation and utilization. The benefits of early detection stemmed from PCPs’ reported objective to prevent disease as well as their knowledge of, and experience with, patients with lung cancer. National recommendations and guidelines for clinical practice issued by the Preventive Services Task Force and the National Comprehensive Cancer Network were also identified as contributing factors that influenced perceptions of screening. One PCP stated,
I'm of the attitude that there's a national body of experts who are far more versed on the literature and spend their career looking at this stuff and spending time looking at and sleuthing through the data and making recommendations. For me to ignore that takes a bit of hubris.
The lung cancer screening process involves multiple, interrelated clinic workflow processes ranging from identifying eligible patients for LCS, initiating shared decision making interactions with patients, referring patients for the low-dose CT, monitoring patients through the process, and following-up with patients post low-dose-CT. To prompt lung cancer screening, a minority of participants had helpful structured workflow processes, like electronic health record (EHR) reminders, to identify eligible patients. The majority of participants stated that they relied on their memory, clinical knowledge, and knowledge of the patient as a reminder to discuss screening rather than a specific clinical process. One participant stated:
I guess I'm old school and I still rely on [memory of eligibility criteria]. I've not totally abandoned that for the EHR and the tickler. I suspect at some time, once it’s more facile with the EHR and how they're building these reminders, maybe I'll find some utility in using them.
Several participants were concerned that patients would be nonadherent to follow-up recommendations due to the lack of systematic processes. Some described challenges when patients referred for lung cancer screening did not show up for their CT at an outside facility. They reported they relied on their own clinic staff to manually track and follow-up with patients who missed their low-dose CT appointment via time intensive reliance on overdue screening order reports. Several PCPs commented it helped to set personal reminders to follow-up with their patients after the low-dose CT as they felt responsible. They also noted ineffective communication between primary care and radiology could lead to nonadherent follow-up care since PCPs sometimes were not alerted when a patient (had not) received their CT. They reported a structured alert would be helpful.
Our participants offered insights to improve lung cancer screening in relation to rural primary care, noting several opportunities to improve and streamline through workflow processes and dedicated staff (Table 4).
Construct: Networks and Communications—
Shared Decision Making. The PCPs in our sample were aware of the Centers for Medicare and Medicaid Services37 requirement for shared decision-making interactions to help patients decide about lung cancer screening. PCPs shared that they were responsible for initiating these conversations with their patients and that it generally happened during annual wellness visits. Our participants supported some core tenets of shared decision making, but did not describe structured workflows to support the decision making process. Very few used a formal online or article-based decision aid describing the risks and benefits of screening to guide those conversations, such as the one provided by the Agency for Healthcare Research and Quality.38 They cited low levels of health literacy among their patients, difficulty accessing a decision aid through the EHR, transition away from printed decision aids, and not finding a decision aid that they like as reasons for electing not to use one. Some participants volunteered that some decision aids were cumbersome and hard to use. One participant stated,
I think the hard thing about using the decision aid is that patients in our community tend to have lower health literacy, and talking to patients from a screening perspective about things like number needed to treat or here's all the potential outcomes that could occur, it can be challenging because it oftentimes doesn't become real to them until they have something wrong with them.
Not enough time in the primary care visit was identified as another reason for not using a decision aid. One participant stated, “[The interaction is] 3 minutes and its part of a visit where we do 8 million other things.” Another commented:
In reality, I think the amount of time that it does take to have a good shared decision-making [interaction] with somebody around something like this, because it typically opens up a can of worms one way or another. If it’s hard to do that without giving up something else that you're talking about… I think it should be done, but I think it would come at the price of giving up something else.
Smoking Cessation Communication. Similar to conducting shared decision-making for lung cancer screening, participants reported screening conversations were a good opportunity to discuss and document a patient’s smoking behavior, as well as discuss lung cancer screening, but they did not always have structured workflow processes in place or clinical time to do so. However, there was a feeling among some participants that conversations about screening and smoking cessation were inherently different. The lung cancer screening conversation is a response to tobacco use health behaviors, while smoking cessation conversations focus on health promotion and disease prevention to improve health. One participant stated,
My general conversations with patients around smoking cessation asks where are you with it?… Then the conversation…with lung cancer screening is a little bit different. Their smoking is why we are screening them for it, but I think it’s more just like any other screening exam here. This is preventative, this is checking to see if we can find something before it becomes an issue.
CFIR Domain: Outer Setting
Construct: Patient Needs, Resources, and Barriers
When asked about external patient barriers to lung cancer screening, geographic location of the low-dose CT was perceived to be the biggest barrier as “driving far is a pretty tough sell (to rural patients).” PCPs mentioned many patients did not have access to personal transportation. In addition to traveling far distances, a few reported that there exists patients’ “abstract distrust of cities and of big hospitals. I think if [patients] trust [clinicians] in the clinic, anything that we can get done there is better.” Cost-effectiveness and radiation exposure were noted as perceived concerns within their patient population too. For example, 1 participant said,
Of the different cancers that we can screen for, I think lung cancer screening probably does not get the attention that it deserves. I think it is either overlooked or maybe even dismissed because, within this [rural patient] community, there's skepticism as to whether or not it is truly beneficial, whether or not it is cost-effective, if the risk of radiation annually is worth it. I mean these are the things that I've heard from other people.
PCPs reported that patients concern about out-of-pocket costs due to uncertainty of insurance coverage for the scan, combined with concerns about lost wages from taking time off work were a significant barrier to patients’ use of lung cancer screening. Some participants reported that clinics with established programs should incorporate patient education about the program itself. A few PCPs indicated the need for patients to be aware of screening’s purpose and benefit in detecting cancer early when it is more easily treated as that would encourage adherence and uptake. They mentioned using public service announcements such as billboard and social media outlets to increase screening awareness. Others stated that the onus is on providers to educate patients.
CFIR Domain: Implementation Process
Construct: Champions
We asked participants whether a clinic champion instigated or led lung cancer screening implementation in their clinic. Of note, champions are “individuals who dedicate themselves to supporting, marketing, and ‘driving through’ an implementation.”32 A minority of participants practiced in clinics with an established program spearheaded by a program champion, and the champions were seen as key facilitators of screening implementation and uptake. In these cases, the champion self-identified themselves to lead lung cancer screening in their clinic, meaning they initiated talks with leadership, sometimes identified mechanisms within the EHR or other systems to find eligible patients, or educated colleagues. Champions were motivated by the clinic’s patient population, creating an opportunity for local change, and lung cancer screening’s benefit for their community to detect cancer early. One participant said, “If you had to champion for something, it is possible to create local change around that… (If) the champion in the clinics that decide that it (screening) is important, I think the rates are going to go up.”
Participants practicing in clinics without a champion described an environment where clinic leadership (eg, medical director or corporate executive officer of a clinic group) did not visibly support lung cancer screening implementation and some fostered a perception that changes to screening processes could not happen. Participants mentioned the lack of time available to undertake additional duties, such as championing an initiative. In the absence of a clinic-level champion, screening processes were managed at the provider level rather than the clinic or program level. In such cases, participants noted screening can be difficult for individual clinicians to negotiate system-level workflow processes such as preauthorization of a CT scan, preauthorization for Medicaid beneficiaries or activating reminders and tracking in the EHR, without the assistance of decision support tools.
Discussion
Our study assessed facilitators and barriers for PCPs to implement lung cancer screening programs. In contrast to prior studies, we focused on rural settings. Our results suggest screening uptake in rural settings is influenced by: 1) PCP perceptions of screening, and networks and communication, 2) patient needs, resources, and barriers, and 3) implementation process characteristics.
We found that all participants were knowledgeable about the benefits of lung cancer screening in detecting lung cancer and the majority were supportive of this procedure. Other investigators have reported mixed results whether PCPs lack of support and knowledge is a barrier to screening uptake.21,27,39,40 Notably, no participants mentioned the American Academy of Family Physicians 2018 statement not to recommend screening,41 which has since changed to a recommendation in support of lung cancer screening. It is possible that respondents did not mention the recommendation because some may not have been family medicine clinicians.42 We previously reported that among rural Oregon PCPs surveyed in 2020, 87% had referred patients for the low-dose CT.27 These and others’ results along with our current findings suggest that PCPs’ knowledge about lung cancer screening has likely increased in the years since the 2013 USPSTF recommendations were published.43
A recent systematic review that reviewed mainly screening in urban settings,40 found that (lack of) structured programmatic elements of high-quality lung cancer screening strongly influenced patient referral rather than individual-level PCP practice, similar to our findings.22,44,45 Some workflow process improvements could include integration of eligibility checklists and decision support into the electronic health record to help PCPs initiate shared decision-making discussions, which could be particularly helpful for PCPs who are less familiar with a patient. Electronic reminders implemented as standards for eligibility and adherence have been helpful for PCPs in other settings, with a direct impact on enhancing the use of medications, improving the recording of medical diagnosis, immunization rates, and increasing implementation of screenings.46⇓⇓–49 Or PCPs could use a “huddle” sheet that could be fastened to the examination room door to remind PCPs about preventive services with another staff member who could assure it gets completed and highlight abnormal results.22
Indeed, introducing the lung cancer screening process is the initial step of a patient’s experience – ensuring adherence to follow-up recommendations is also critical. Unfortunately, less than a quarter of patients in routine care settings have appropriate adherence.50 The responsibility for adherence is perceived to lie with the PCP in both nonrural and rural settings, similar to participants in our study.21,51 Like a larger survey study of rural-serving PCPs, our participants suggested use of systematic processes like centralized programs and dedicated lung cancer screening coordinators.27
We found that, while all PCPs interviewed reported they engaged in shared decision-making interactions with their patients, they did not rely on or use a decision aid to guide the conversation. Similar to studies in other mainly urban settings, PCPs cited a lack of time as the primary reason for not using a decision aid.40,52,53 One solution to relieve the time pressure of a primary care visit is to redesign clinic workflow processes to involve other clinic staff members. For example, nurses can engage in shared decision-making interactions, and having a dedicated lung cancer screening coordinator has been helpful for PCPs in other studies.39,54,55
While conversations about tobacco use can prompt screening discussions, many clinicians in this and other studies considered conversations about lung cancer screening and smoking cessation to be inherently different and not related.56 This is perhaps because discussions of tobacco use are focused on health behavior change, (ie, primary prevention). Lung cancer screening, rather, is a secondary prevention conversation about identifying lung cancer in the earliest stages. Clinicians and patients may feel that there are other motivators for changing smoking behaviors beyond screening, or there may not be enough emotional distress involved within discussions to lead to a “teachable moment” to elicit a health behavior change.56 PCPs may be able to leverage other motivations and aspects of communication, such as building trust within the patient-clinician relationship, to improve motivation for cessation within lung cancer screening discussions. Discussing the importance of screening as only one part of prevention, or discussing risk lung cancer may be helpful to personalize discussions. PCPs may also need to include postscreening interventions, or interventions beyond the commonly used techniques like the 5A’s.57
PCPs shared that rural patients face many persistent barriers to lung cancer screening. One previous qualitative study from 2019 interviewed 10 PCPs in New Mexico who provided care to underserved populations.61 The participants did not use low-dose CT screening and were skeptical of the evidence-based behind lung cancer screening. This is different from our study since our participants endorsed the evidence base and were aware of the benefits of using low-dose CT vs other modalities. Our findings support the importance of addressing patient-level barriers to screening that are unique to rural populations and have been shown to diminish patient access and utilization of screening.58,62,63 Possible solutions that were not mentioned in our study to mitigate transportation barriers include mobile CT scanners,64,65 shared decision-making interactions conducted via telehealth,66 increasing awareness of federal cancer centers,59 and round-trip transportation vouchers67 to a qualified medical center for the low-dose CT scan. Lastly, health navigators who provide high-quality communication60,68,69 and are positioned to support patients through the screening process can help to respond to barriers and address mistrust and health literacy issues among rural-residing patients.60,70,71
System-level facilitators for implementation include the usage of champions who are known for facilitating change efforts in health care settings.72 Our results similarly suggest primary care champions are necessary to create change and obtain resources by elevating the importance of screening. Importantly, lung cancer screening champions do not always have to be a clinician. To identify champions, the Institute for Healthcare Improvement suggests the following: seeking volunteers rather than formally appointing a staff member, giving the champion power to implement solutions, having leadership remind other staff that the champion is not a disciplinarian but is there to help, training the champion on Human Factors Engineering (which identifies why people make mistakes), integrating the champion within other disciplines to learn from each other, and checking with staff to assess ongoing implementation processes.73 Although participants in our study noted the lack of available time to champion initiatives, there may be opportunities for clinics to appoint a staff member to champion multiple wellness or prevention initiatives.
Limitations
While this study is one of a handful that qualitatively examine rural PCPs’ perceptions of factors related to lung cancer screening implementation, it has limitations. First, we may have obtained a biased sample of PCPs who were more knowledgeable about screening and more likely to volunteer to be interviewed. However, as our cohort was diverse in other ways, we believe the information gathered can be extrapolated to reflect barriers and concerns among most PCPs practicing in other rural regions of the United States serving similar patient populations. Second, there were refusals due to COVID-related strain that made recruitment challenging; we could not determine the percentage who responded to the study invitation, and we could not assess for differences compared with nonrespondents. However, the goal of qualitative research is not to be representative, but instead to give voice to participants with knowledge of the topic. Third, we did not assess the quality of local programs in the regions for each participant or based on the setting in which they practice (eg, independent or system affiliated). Future work should explore if clinician views toward lung cancer screening vary based on regional program quality or clinician type. Fourth, our participants were not racially or ethnically diverse, reflecting the demographics of Oregon PCPs, but limiting generalizability.74 Finally, we did not conduct interviews with patients and therefore our patient perspective is from a clinician lens.
Conclusion
We found that rural-practicing PCPs were supportive of lung cancer screening and discussing it with patients, although workflow processes, time challenges, and patient-reported barriers remain impediments to improved lung cancer screening in their clinics. PCP participants described how rural patients have accessibility and referral needs that need to be addressed. Overall, our findings point to the need for clinic- and system- level tools to improve lung cancer screening uptake.
Acknowledgments
We would like to acknowledge Julia Mabry, Sarah Bumatay, and Tara Thomas for their help with this study.
Notes
This article was externally peer reviewed.
Funding: This project was supported by the OHSU Knight Cancer Institute NCI Cancer Center Support Grant P30CA069533. It was also supported by resources from the VA Portland Health Care System, Portland, Oregon. Dr. Davis’s time was supported in part by a career development award from the National Cancer Institute (1K07CA211971-01A1).
Conflict of interest: CGS serves in several administrative roles for lung cancer screening program in the Veterans Health Administration and does not receive financial compensation for that role. He has no professional affiliation with any study participant. He served on the American Lung Association panel to develop an online toolkit to support lung cancer screening efforts and received no financial compensation for that role. The author authors have no conflicts of interest to disclose.
Disclosure: The Department of Veterans Affairs did not have a role in the conduct of the study; in the collection, management, analysis, or interpretation of data; or in the preparation of the manuscript. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S. Government.
To see this article online, please go to: http://jabfm.org/content/36/6/952.full.
- Received for publication March 20, 2023.
- Revision received May 31, 2023.
- Accepted for publication June 5, 2023.