Abstract
Background: Despite antiviral agents that can cure the disease, many individuals with Hepatitis C Virus (HCV) remain untreated. Primary care clinicians can play an important role in HCV treatment but often feel they do not have the requisite skills.
Methods: We implemented a population-based improvement intervention over 10 months to support treatment of HCV in a primary care setting. The intervention included a decision-support tool, education for clinicians, enhanced interprofessional team supports, mentorship, and proactive patient outreach. We used process and outcome measures to understand the impact on the proportion of patients who initiated treatment and achieved Sustained Virologic Response (SVR). We used physician focus groups and pharmacist interviews to understand the context and mechanisms influencing the impact of the intervention.
Results: Between December 2018 and June 2020, the percentage of HCV RNA positive patients who started treatment rose from 66.0% (354/536) to 75.5% (401/531) with 92.5% (371/401) of those starting treatment achieving SVR. Qualitative findings highlighted that the intervention helped raise awareness and confidence among physicians for treating HCV in primary care. A collaborative team environment, education, mentorship, and a decision-support tool integrated into the electronic record were all enablers of success although patient psychosocial complexity remained a barrier to engagement in treatment.
Conclusion: A multifaceted primary care improvement initiative increased clinician confidence and was associated with an increase in the proportion of HCV RNA positive patients who initiated curative treatment.
- Family Medicine
- Focus Groups
- Hepatitis C
- HCV Antibodies
- Pharmacists
- Primary Health Care
- Physicians
- Quality Improvement
- Sustained Virologic Response
Introduction
Hepatitis C Virus (HCV) affects 58 million people worldwide.1 Chronic infection leads to liver complications, including cirrhosis and carcinoma, and can reduce life span by as much as 15 years.1 In 2019, HCV resulted in approximately 1.1 million deaths globally.1 Even in high-income countries, HCV has resulted in more years of life lost than other infectious diseases including HIV, influenza, and pneumococcal disease. These deaths are particularly distressing because they are avoidable.2
Hepatitis C can be cured. Curative interferon-based treatment agents became available in 1998, but had significant side effects. Direct-acting antiviral (DAA) agents have been available since 2014. A simple, 2 or 3-month treatment regimen with these oral agents cures 95% of patients with minimal side effects.3 But, despite the availability of these new drugs, according to World Health Organization data approximately 6 out of 10 people with HCV remain untreated worldwide1. Reasons for being untreated are multifactorial but include financial barriers, lack of awareness, fear of side effects, difficulty with adherence and comorbid conditions, including substance use.4
HCV treatment has historically been the purview of internists specializing in hepatology or infectious disease. However, DAAs are relatively straightforward to prescribe and use, opening the door to more community-based treatment—a particularly relevant approach given that many individuals who remain untreated in high-income countries struggle with social issues including incarceration and substance use.5,6 Family Medicine specialists are uniquely situated to treat HCV because of their longstanding relationships with patients, and ability to connect with hard-to-reach populations. However, many do not feel they have the knowledge and skills to initiate HCV treatment.7
We designed a quality improvement initiative to empower and support Family Medicine specialists to cure Hepatitis C using a data-driven, planned, proactive approach for their practice population. We tracked process and outcome measures to understand the implementation and impact of the initiative and used qualitative methods to understand the context and mechanisms that influenced project outcomes.
Methods
Setting and Context
The St. Michael’s Hospital Academic Family Health Team (SMHAFHT) serves approximately 49,000 patients at 6 primary care clinics in the inner city of Toronto, Canada. The team cares for many historically marginalized populations including people with HIV, mental health conditions and addictions, and those living in poverty. The team comprises approximately 80 staff physicians and more than 60 other health professionals including nurses, nurse practitioners, pharmacists, and social workers.
The vast majority of our patients are permanent residents of Ontario and primary care services are free at the point-of-care via the Ontario Health Insurance Plan (OHIP) but medication coverage is varied. DAA therapy can range in cost from CAD$45,000 to CAD$100,000. Some patients have coverage via private insurance and others via Ontario Drug Benefits (eg, if they are on social assistance, age 65 and older, or meet low-income criteria). Those without prescription drug coverage can access drug company special access funds. DAAs for HCV became fully covered for recipients of Ontario Drug Benefits in 2018. Data drawn from our practice electronic medical record (EMR) in 2017 found only 47% of those who were HCV RNA positive were engaged in treatment.7
Intervention
We conducted a multi-faceted, population-based quality improvement intervention between March 2019 and December 2020 to increase HCV treatment rates. First, we supported our clinical team to develop the knowledge, skills, and processes to treat HCV within the Family Medicine setting. We worked with a clinical working group to develop an internal treatment pathway that summarized work-up and treatment of HCV, team roles, and other supports (Online Appendix 1). One pharmacist with HCV expertise trained the 3 general pharmacists on our team so that all had expertise in HCV medication management. One pharmacist (DC) continually updated the medication algorithm available in the decision-support tool form based on evolving guidelines. We identified 4 physician peer mentors with experience treating HCV who could be available for support. We also worked closely with 2 hepatologists who reviewed our pathway and tools and who were available to be consulted for complex cases. In collaboration with our EMR specialist, we built an interactive HCV decision support tool for the EMR to guide workup, treatment, and consultation with peer mentors, pharmacists, and specialist (Online Appendix 2). Between March and June 2019, we delivered group education sessions to clinic staff at departmental rounds and a lunch-and-learn session at each of the 6 clinics. The EMR tool was proactively entered on the charts of patients with designated HCV positive.
Second, we conducted proactive outreach for all registered practice patients known to be HCV RNA positive or whose HCV treatment status was unknown. We identified patients who were HCV antibody positive using an automated search of our electronic medical record (EMR) and manual chart review by a trained research coordinator (ACN) as done with a previous chart audit.7 Between May and August 2019, research staff met individually with physicians with at least 1 patient known to be HCV RNA positive for more than 6 months to provide education on the treatment pathway and the decision support tool and to confirm their list of active untreated patients. Physicians confirmed HCV status and determined whether a patient should be contacted on their behalf. A clerical staff member called these patients (Online Appendix 3). If we were unable to reach them after 2 attempts, we sent a letter and/or e-mail. Initial outreach was done for all patients between May and September; a second round was done between November and December 2019.
The intervention was refined iteratively based on feedback from a clinical working group and a patient advisory group as well as review of process and outcome measures by the study team.
Evaluation Approach
We conducted a mixed method evaluation excluding patients who died or left our practice during the study period. First, we tracked outcome measures for all registered practice patients who were HCV antibody positive (% who started treatment; % with sustained virologic response [SVR]) and process measures for those patients eligible for proactive outreach (% with an informed discussion, % with some pretreatment work-up). Data were collected by ACN using manual chart audit every 4 to 8 months.
A second team member reviewed twenty of the charts to ensure HCV treatment status and outcome measures were correctly classified. There were no discrepancies between the 2 reviewers. We did not do serial reviews of the charts of patients who were successfully treated or who had spontaneously cleared the virus at baseline.
Second, we compared characteristics of HCV RNA positive patients who were, and were not, treated at the end of study using Chi square and Mann-Whitney tests. Data on patient demographics, comorbidities, and visit history were collected using an automated search of our EMR. Age, sex, and postal code were collected from registration information. Postal code was used to derive neighborhood income quintile using 2007 census data from Statistics Canada. We noted whether patients had HIV, diabetes, or serious mental illness based on ICD codes validated by the most responsible clinician; we used diagnostic and service codes from physician billing to note whether patients had any mental health condition or addiction. We used billing data from EMR to determine the number of visits in the last year. These analyses were done using R Version 4.0.0.
Finally, we explored physicians’ and pharmacists’ perceptions of the strengths, limitations, contextual contributors, and perceived outcomes of the intervention. We conducted 3 focus groups with staff physicians, targeting those who had patients eligible for proactive outreach (n = 65), and individual interviews with pharmacists (n = 4). Invitations and reminders were sent by e-mail. Focus groups and interviews were held in person or online, were conducted by an experienced qualitative researcher, and were audio recorded and transcribed verbatim; they were 30 to 60 minutes in length and followed a semistructured guide developed by the research team (Online Appendix 4).
We analyzed the qualitative data after interpretive description,8 which involved reading the transcripts, describing the main patterns related to the research questions, and interpreting the results with the full team (including clinicians and methodologists). After best practices in program evaluation9 and implementation science,10,11 we focused on aspects of both the intervention and the context that may have influenced outcomes. KH inductively coded the data to explore participants’ general impressions of the initiative and then grouped the codes into program strengths or limitations, contextual facilitators or challenges, and perceived outcomes. KH was not involved in the design or implementation of the QI initiative, enabling an openness to the data during inductive coding. KH iteratively discussed the codes and categories with CJP, AS, and TK, who provided methodological, clinical, and content expertise in the later stages of analysis. We also explored patients’ perspectives on receiving HCV treatment, but report on those separately.
Clinician and Patient Involvement
A clinical working group helped inform the intervention, engage clinicians, and interpret results. In addition, an advisory group of patients with lived experience of HCV gave advice on the intervention, particularly our approach to patient outreach, and the interpretation of the results. As an example, based on advice from patient advisors, we increased health promotion efforts about HCV treatment through social media and posters in community pharmacies and clinics using material from a reputable national organization (https://orders.catie.ca/publications/treatment/).
Ethics
Institutional authorities at Unity Health Toronto formally reviewed a protocol for the quantitative component and deemed it to neither require Research Ethics Board approval nor written informed consent from participants. The qualitative study protocol was reviewed and approved by the Unity Health Toronto Research Ethics Board.
Results
We identified 701 patients who were HCV antibody positive in December 2018, 536 of whom were HCV RNA positive and 165 that had spontaneously cleared the virus (Figure 1). Of those who were HCV RNA positive, 299 were fully treated successfully, and 55 were actively engaged in treatment at the start of our initiative. We identified 182 patients who were HCV RNA positive and potentially still needed HCV treatment (ie, treatment naïve, had unknown treatment status, or previous treatment failure).
Cohort of patient identified as being Hepatitis C Virus (HCV) RNA positive as of December 2018 and needing treatment.
Patient Characteristics
The average age of patients needing HCV treatment was 52.4 years; 64.3% (n = 117) were male, 20.7% (n = 18) lived in a neighborhood in the lowest income quintile, 14.9% (n = 13) were homeless, 70.9% (n = 129) faced challenges with mental health or substance use, 16.5% (n = 30) had HIV. The mean number of physician visits for these patients was 12.9 over a 1-year period (Table 1).
Characteristics of Patients Included in the Intervention Who Were Hepatitis C Virus (HCV) RNA Positive for More Than 6 Months and Untreated1 as of December 2018 (n = 182)
Patient Outreach
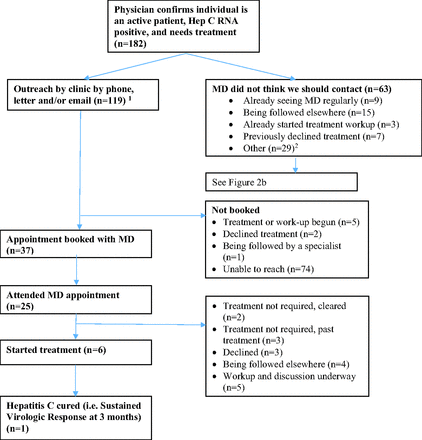
Of the 182 patients eligible for outreach, 119 were contacted by phone, e-mail or letter (Figure 2a). 74 of the patients who were contacted did not respond to calls, had no e-mail consent on record, and/or their address was no longer current. We reached 45 patients, of whom 37 were booked with their physician, 25 attended their appointment, and 6 ultimately began treatment.
Individuals who were Hepatitis C Virus (HCV) RNA positive and untreated in December 2018 and received study outreach. Sixty-three were called twice; 54 letters and 3 emails were sent. Other reasons include mental health issues, complicated or difficult patients, pregnancy, cancer, incarceration, not engaged in care or unknown.
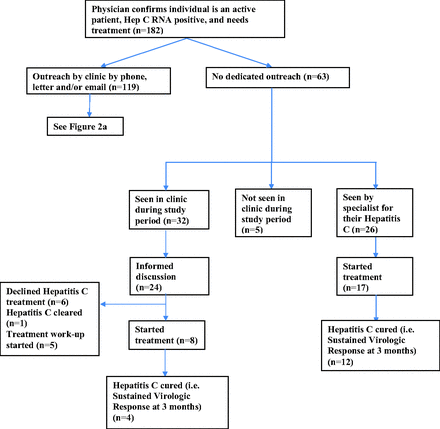
Individuals who were Hepatitis C Virus (HCV) RNA positive and untreated in December 2018 but did NOT receive study outreach.
Physicians declined patient outreach for 63 patients of the 182 eligible for outreach, citing a variety of reasons including prior engagement in treatment work-up, being followed elsewhere, had previously declined treatment or were already seeing the MD regularly. By the end of the intervention, 26 of these 63 patients had been seen by a hospital-based internist, hepatologist or infectious disease specialist to discuss HCV treatment. Another 32 of the 63 were seen in our family practice for other reasons, and of these, 24 had an informed discussion with their Family Medicine specialist about HCV treatment (Figure 2b).
Outcome Measures
Between December 2018 and June 2020, the percentage of HCV RNA positive patients who started treatment rose from 66.0% (354/536) to 75.5% (401/531). Similarly, the percentage of HCV RNA positive patients who achieved SVR rose from 55.8% (299/536) to 69.9% (371/531). As of June 2020, 92.5% (371/401) of patients who started treatment achieved SVR (Figure 3).
Change in the number of Hepatitis C Virus (HCV) RNA positive patients who started treatment and achieved cure (Sustained Virologic Response, SVR) between December 2018 and June 2020.
Process Measures
Of the 182 HCV RNA positive patients untreated in December 2018, 60.4% (110) had an informed discussion with their doctor regarding HCV treatment. 25.3% (46) had some pretreatment investigations including repeat HCV RNA level or an ultrasound ordered by their family doctor. 9.9% (18) declined treatment at various stages in the process, 9 declined before an appointment was booked with their clinician and 9 declined after having an informed discussion with their clinician. The EMR decision support tool was used by 29 unique physicians on 45 patients.
Characteristics of Individuals Who Have and Have Not Been Treated
At study end, there were 130 HCV RNA positive individuals who still required treatment (Table 2). Compared with those who were treated, individuals who were untreated were younger (51 vs 57, P < .001), a greater proportion were women (35.4% vs 25.4%, P < .05), lived in the lowest neighborhood income quintile (24.2% vs 4.1%, P < .001), and had a comorbid diagnosis of mental health or addiction (73.9% vs 62.8%, P < .05). Mean visits were lower among those who were untreated (8.1 vs 10.8, P < .001); specifically, a higher proportion of those untreated had zero visits to a physician during the study period (33.1% vs 10.6%, P < .001).
Characteristics of Patients Who Were Hepatitis C Virus (HCV) RNA Positive for >6 Months, by Whether or Not They Were Treated at the End of the Intervention
Qualitative Findings
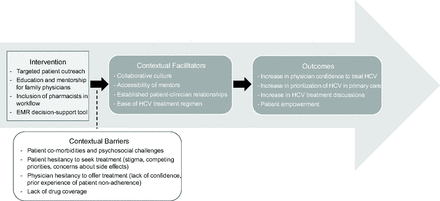
Between February 2020 and June 2021, we held 3 focus groups with a total of 14 physicians and 3 individual interviews with pharmacists, representing clinicians across 4 of 6 clinical sites. Findings are summarized below and summarized in Figure 4.
Facilitators and barriers influencing outcomes after the intervention, summary of qualitative findings from physician focus groups and pharmacist interviews. Abbreviation: HCV, Hepatitis C Virus.
Program Strengths and Contextual Facilitators
Participants highlighted key strengths of the initiative, including the EMR-embedded decision support tool, team-based approach, mentorship, and the multi-pronged educational support. Collectively, these supports reportedly increased physicians’ confidence in HCV treatment and alleviated their need to research pharmacological aspects of treatment, which they indicated was previously a barrier. Many noted that the decision support tool was critical to the program’s success and sufficient as a stand-alone support, but that the education and mentorship helped to build comfort in using the tool initially.
Participants conveyed that the collaborative culture of the practice and the accessibility of the mentors were key facilitators, often enabling just-in-time patient care. Participants spoke highly of the pharmacist-physician collaboration:
Because the pharmacist was profiled or highlighted in multiple parts of the work… I think [it]… gave clinicians the understanding that the pharmacist was there as a support for them. (Pharmacist 3)
I feel like why [the initiative] worked well here was because of the really easy access to [the HCV] team. We're lucky because we have the pharmacist in house, so even if it wasn't an instant message through EMR, it was a tap on the shoulder when they're walking by. And sometimes that makes all the difference, actually, because if a patient who's there, who may not follow up. … Sometimes it has to happen at that moment. (Physician 2)
In addition, the established patient-clinician relationships and the relative ease of the current HCV treatment regimen appeared to facilitate patients’ acceptance and uptake of the treatment.
Program Limitations and Contextual Barriers
Physicians spoke about challenges with patient adherence and concerns related to potential reinfection. Despite social work supports, clinicians described psychosocial complexities as a barrier to treatment including mental health and addictions comorbidities and housing instability:
We’re still trying to figure out how exactly to increase the continuity with these patients. Most of my patients have a drop-in policy, so they just come in, they’ll see me right away. But even that, I don’t think, is enough to ensure that they’ll be compliant with the entire course of medication. … That’s probably where maybe some advice from our clinical champions to figure out how to actually work with this patient population. (Physician 5)
They’re a hard to reach bunch sometimes because there’s often concomitant illnesses, mental illness or addiction. (Physician 6)
Some clinicians spoke of specific instances in which they tried to initiate treatment, but patients did not follow through, whereas others noted cases where they assumed adherence would be an issue so did not offer treatment. Notably, a few physicians described forgoing treatment conversations with patients because of worries about adherence:
There are some patients that I personally didn't think would succeed in treatment, and they did, and they may have failed at other times. I think I just had this pre-conceived notion of other patients in the past that maybe I shouldn't offer it… that it's possible that we that might be our own filter. And sometimes we get in the way of our own opinion of whether people really could, or at least minimally should be offered and given a chance to [accept treatment]. (Physician 2)
Some emphasized the importance of offering treatment multiple times because some patients accept after previously refusing. Patients’ concerns about potential side effects and lack of drug coverage were additional barriers to treatment which clinicians felt was out of their control.
Our interviews with patients [data not presented] corroborated the views of clinicians, with patients noting that competing health priorities and life circumstances, as well as concerns about side effects, prevented them from initiating treatment.
Program Outcomes
Physicians found that the initiative raised their awareness of HCV, and prioritized it, enabling some to overcome their hesitancy to treat:
I had become practiced in probably not addressing [HCV] as assertively as I should have because I presumed I knew the answer in a couple of cases… I think it brings to light the idea that, at some point, these patients probably will change their minds and we just need to be opportunistic in terms of grabbing them at the right stage of their life. (Physician 4)
Participants indicated that the program increased physicians’ confidence in treating HCV and empowered them to view treatment as within their scope of practice:
The message that this initiative gave is that [treating HCV] is something within your competency. You can do this safely as a family doctor. Sometimes we kind of question ourselves like, “Should I be sending this person out to a specialist? Am I going to be able to do a good enough job?” And so the fact that the message was clear: Yes, this is something you can do; here's how you do it. I think it was an enabler for us. (Physician 14)
Participants also noted that treating HCV may have helped their patients feel empowered by enabling them to gain control of their condition:
I've had two patients who … were actually quite emotional about being treated and feeling a sense of autonomy or being able to take charge of something with regard to their health… this was, I think, really empowering for them. (Physician 1)
I think the patient and myself were both so excited getting that final negative RNA. It was like the best thing in the world. (Physician 11)
Discussion
Our multifaceted quality improvement initiative resulted in an overall increase in patients initiating HCV treatment from 66% to 76% with 93% of those initiating treatment achieving a SVR by the end of the 18-month study period. Further, nearly 2/3 of patients who were untreated at the start of the study had informed discussions about HCV treatment with their clinicians by study end. Compared with those who were treated, a higher proportion of patients who were untreated were younger, female, had comorbid mental health and addictions, and had zero physician visits during the study. Qualitative findings highlighted that the intervention helped raise awareness and confidence among physicians for treating Hepatitis C in primary care. A collaborative team environment, education, mentorship, and a decision-support tool integrated into the electronic record were all enablers of success although patient psychosocial complexity remained a barrier to engagement in treatment.
Our initiative focused on patients of our large primary care organization. Even so, we were unable to reach almost 2/3 of untreated patients who we attempted to proactively contact by phone, letter, and/or e-mail. For some patients, the contact information was not up to date, whereas others may not have had a working phone or stable housing—a reflection of the challenging social circumstances facing many people with Hepatitis C and the opportunity for primary care organizations to take more creative steps to retain them in care. Indeed, we found higher treatment rates among those HIV in our practice likely reflecting their greater engagement in ongoing care. The vast majority of untreated patients who did not receive active outreach from our team were engaged in care during the study period either with an HCV specialist or with their Family Medicine specialist.
That untreated patients were more likely to have mental health and addictions may speak to these patients having different care priorities for themselves, but our qualitative results suggest it may also relate to physicians making a judgment that a patient will be unable to successfully engage in treatment. Studies have shown, however, that SVR is achievable, even in this patient population.4,5,12⇓–14 Specifically, people who use drugs can be effectively treated for HCV with supports such as telephone reminders,13 coverage of transportation costs,15 engagement with community workers and patient navigators,16,17 and help with applications for drug coverage.17,18 Other strategies for supporting adherence among patients with psychosocial complexity include medication delivery, connection to opioid use disorder treatment,19 enhanced pharmacist care13,16,20 or incentives such as grocery cards.21 Many of these supports were available to our patients but we hypothesize that they were not used systematically and that clinicians would benefit from additional education on strategies to support more marginalized populations. Notably, there was a relatively high prevalence of schizophrenia and bipolar disorder among our patients with HCV—a finding in keeping with the literature.22
Our results are in keeping with studies that have shown family medicine teams can effectively treat HCV infection using direct acting antiviral therapy in settings as diverse as rural Australia,16 Alaska,23 and the US inner city.4,5 Indeed, studies have shown very similar cure rates13,23⇓–25 between hospital and family medicine settings. Empowering primary care to treat HCV is particularly relevant given the importance of positive patient relationships and trust in supporting marginalized populations.15 Our initiative integrated many strategies shown to be effective for spreading HCV to primary care settings including coordinated care with other health care disciplines such as case managers, nurse practitioners, and pharmacists, patient outreach, designating leaders or champions within the team4,16,23,25,26—strategies that could be spread to other primary care teams.
Our study has both strengths and limitations. We implemented a population-based multifaceted improvement initiative to increase HCV treatment rates largely using existing practice resources—the only such initiative to our knowledge in Canada. We conducted a mixed method evaluation that demonstrated practice-level improvement in our primary outcome and included qualitative interviews that provided insights into the context and mechanisms influencing outcomes, including ones that are hard to measure. However, our study was conducted at a single primary care organization in an urban area that serves a relatively high number of patients with HCV and includes government funding for different health professionals including pharmacists, nurses, and social workers—factors that limit its generalizability. Further, our study began just after direct-acting antivirals became covered by the provincial drug formulary and it is difficult to speculate how many people would have been treated in the absence of our intervention. However, our qualitative findings support that our intervention positively influenced clinician behavior.
Conclusion
A multifaceted quality improvement initiative implemented in a primary care setting over 10 months using existing team resources was associated with an increase in the proportion of patients with RNA positive HCV who had initiated curative treatment. Clinicians described improved awareness and confidence in treating HCV after the intervention with key enablers being the collaborative culture, team supports, mentorship, education, and a decision-support tool. The working relationship between team pharmacists and physicians was a key strength. Potential next steps include spreading this intervention to other family practices although doing so may require funding for team resources, particularly pharmacists. Future iterations of the intervention will also need to include strategies to directly address the psychosocial complexity of patients—a real and perceived barrier to treatment. Our study adds to the growing literature demonstrating that with the right supports, Family Medicine specialists can play an important role in the global pursuit of HCV elimination.
Acknowledgments
We are grateful to Aine Workentin who assisted with physician education; Erin McAllister, who completed the patient outreach; Lisa Miller, who helped develop the decision-support tool embedded in the electronic medical record; and Mo Al-Haj, who conducted much of the data extraction using automated searches of our electronic medical record. Thank you to our team pharmacists Jon Hunchcuk, Brenda Chang, and Doret Cheng as well as their hospital colleague Mark Naccarato and liver specialists Dr. Hemant Shah and Dr. David Wong, who assisted with the development of the study materials and provided guidance to the family doctors treating their patients with Hepatitis C. Thanks also to Tiffany Jenkins of the Applied Health Research Centre (AHRC) and Tony Antoniou for their support and contribution to the qualitative research component. Finally, thank you to clinical working group members not already mentioned, including Michael Adia, Yosra Al Makadma, Daniel Bois, Ashna Bowry, Monica Gad, and Zoe Von Aesch, who provided overall guidance on the project. Much appreciation goes to our patient advisory group including Neil Davis, Walter Tupholme, Jay Wagner, Helen Posno, and Andrew Cumming, who provided input on the study including improving patient outreach and our patient interview guide.
Appendix 1.
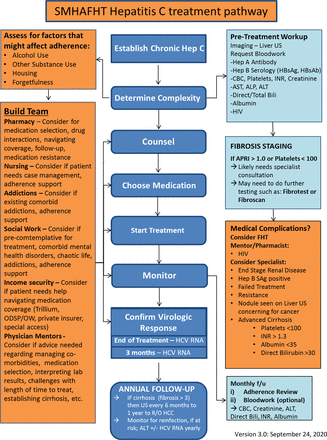
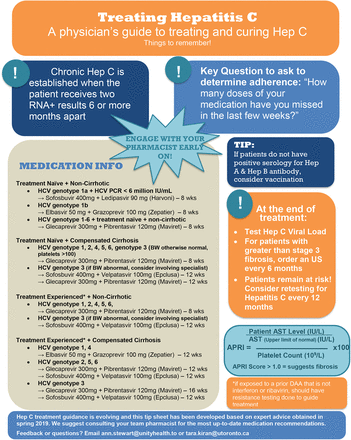
Appendix 2.


Appendix 3.


Appendix 4.



Notes
This article was externally peer reviewed.
Funding: This study was supported by a grant from Gilead Sciences Canada and from the St. Michael’s Hospital Foundation. The study sponsors had no role in study design, data collection, analysis, interpretation of data, manuscript preparation, or the decision to submit for publication.
Conflict of interest: Drs. Ann Stewart and Tara Kiran were co-PIs on the study grant from Gilead Sciences Canada, a company that produces medications to treat Hepatitis C. However, the study sponsors had no role in study design, data collection, analysis and interpretation of data, manuscript preparation, or the decision to submit for publication.
Dr. Gordon Arbess has received honoraria for presentations from Gilead Sciences Canada and Merck. He sits on the Advisory Board for Gilead Sciences Canada, Merck, and Viiv Healthcare. The other authors have no conflicts of interest to declare.
To see this article online, please go to: http://jabfm.org/content/36/4/591.full.
- Received for publication December 22, 2022.
- Revision received March 6, 2023.
- Accepted for publication March 13, 2023.