Abstract
Objective: To create a model based on patients’ characteristics that can predict the number of burdens reported using the ICAN Discussion Aid, to target use of this tool to patients likeliest to benefit.
Patients and Methods: Six hundred thirty-five patients (aged ≥18 years) completed the ICAN Discussion Aid at a Scottsdale, Arizona, family medicine clinic. Patient characteristics were gathered from their health records. Regression trees with Poisson splitting criteria were used to model the data.
Results: Our model suggests the patients with the most burdens had major depressive disorder, with twice as many overall burdens (personal plus health care burdens) than patients without depression. Patients with depression who were younger than 38 years had the highest number of personal burdens. A body mass index (BMI) of 26 or greater was associated with increased health care burden versus a BMI below 26.
Conclusion: The number of burdens a patient will report on the ICAN Discussion Aid can be approximated based on certain patient characteristics. Adults with major depression, a BMI of 26 or greater, and younger age may have greater reported burdens on ICAN, but this finding needs to be validated in independent samples.
- Arizona
- Behavioral Medicine
- Burden of Illness
- Caregiver Burden
- Chronic Disease
- Clinical Medicine
- Communication
- Family Medicine
- ICAN Discussion Aid
- Mental Health
- Patient-Centered Care
- Shared Decision-Making
Introduction
According to 2021 data from the Centers for Disease Control and Prevention, 6 in 10 Americans have at least 1 chronic health condition, and 4 in 10 have 2 or more chronic conditions.1 Managing a chronic condition requires work by the patient, such as attending health care appointments, self-monitoring, adhering to a medication regimen, and planning and adhering to a special dietary regimen. This workload comes in addition to symptom burden and the challenges of daily life, which can leave health care work undone.2 Uncompleted health care work can lead to worsening patient outcomes, which then are met with intensifying treatment, creating a vicious cycle of infeasible work and unmet symptom burden.2
Increasingly, health care clinicians have recognized that treatment plans must fit realistically into a patient’s daily life, but because each person’s lived experience is complex, clinicians may forgo problem-solving any issues of fit.3 In 2016, the ICAN Discussion Aid was developed to help clinicians and patients contextualize care for the individual patient.4 This discussion aid allows patients to identify parts of their life, including health care, that are sources of burden or satisfaction and then review the results with their clinician.4 Working together, the patient and clinician can then form feasible plans of care. A pilot study of ICAN in 2019 showed that the discussion aid promoted the discussion of important health care topics without adding to the visit length5; a larger study is under way.
ICAN was created to use with patients with any chronic condition, and clinicians can implement the aid to fit their clinic’s population and workflow.5 Although this intervention is highly adaptable to different settings, which patients may benefit most from its use remains unknown. In implementation work, the aid’s developers in the Knowledge and Evaluation Research Unit have seen resource-limited settings restrict using ICAN to subsets of patients for whom they perceive the need. These methods have not been validated and may overlook some patients who could benefit.
To our knowledge, no studies to date have examined patient characteristics that are associated with more burdens reported on the ICAN Discussion Aid with the number of burdens standing as a marker of potential benefit from using this tool in practice. Therefore, the aim of this study was to examine the possibility of modeling the greatest number of personal and treatment burdens predicted by known patient characteristics. This knowledge may help identify which patients would benefit the most from using ICAN to improve the fit of their care plans.
Patients and Methods
Intervention
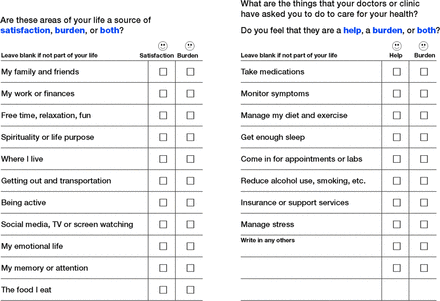
The ICAN Discussion Aid asks patients to consider whether they are satisfied/helped or burdened by specific areas of their personal lives and their experience with health care (eg, family and medications) (Figure 1).5 Finally, it asks 3 open-ended questions: (1) “What are you doing to manage your stress?” (2) “Where do you find the most joy in your life?” and (3) “What else is on your mind today?” (Appendix Figure 1). After completing the ICAN Discussion Aid, the patient and clinician review the tool together, first discussing the 3 open-ended questions and then the remainder of the discussion aid. Clinicians prompt patients to elaborate on their experiences, typically by asking, “What stands out to you from your answers?” This tool was developed with funding from the Agency for Healthcare Research and Quality and is freely available at https://carethatfits.org/ican.
ICAN Discussion Aid. Inside bifold of flyer lists selections for sources of satisfaction and burdens. Labs indicates laboratory tests. (Used with permission of Mayo Foundation for Medical Education and Research)
Study Participants
This retrospective cross-sectional modeling study was approved by the institutional review board. The study cohort comprised a convenience sample of 678 consecutive patients (aged ≥18 years) who came for office visits with a single primary care clinician at a family medicine outpatient clinic in our region, from April 1, 2018, through May 31, 2021. Of the 678 eligible patients, 635 patients consented to complete the ICAN Discussion Aid as a part of the rooming process. A registered nurse introduced a paper-based ICAN to the patients, and they were prompted to complete it while waiting for their clinician to enter the room.
Retrospective Review
From June 1, 2021, through July 31, 2021, we performed a retrospective review of the electronic health records (EHRs) of patients who completed the ICAN Discussion Aid. Patient identifiers were sorted in numeric order without respect to their responses on ICAN to avoid bias during the EHR review. Independent variables were chosen based on common chronic conditions treated in the practice and manually gathered at EHR review. Table 1 reports the variables extracted.
Patient and Clinical Characteristics
Statistical Analysis
Descriptive statistics were used to summarize patients’ demographic and clinical characteristics and responses on the ICAN Discussion Aid. The total number of burdens was defined as the number of items in which the respondent answered “burden” (that the variable was a burden to them) or “both” (both a burden and an area of satisfaction). ICAN questionnaires that were completely blank were excluded in the final analysis, but we did include questionnaires that were partially completed if the patient made any markings. In those instances, individual blank responses or ambiguous markings were not considered burdensome.
We then fit regression trees using rpart6 (R Foundation for Statistical Computing) with a Poisson splitting criterion to model the most predictive factors associated with a high number of patient-reported burdens. Our goal was to develop a model for predicting the number of burdens based on patient characteristics. We developed 3 separate models: (1) overall burdens (sum of the numbers of personal and health care burdens), (2) the number of personal burdens (eg, at home and work), and (3) the number of burdens related to health care. Appendix Figure 2 shows a histogram of the number of burdens (personal, health care, and overall). These 3 variables had a high proportion of zeros and were right-skewed, which violated the assumptions of many classic statistical modeling techniques. Therefore, a regression tree using a Poisson splitting criterion for count data was chosen to model the number of burdens. R Statistical Software version 4.0.3 (R Foundation for Statistical Computing) was used to conduct the analysis.
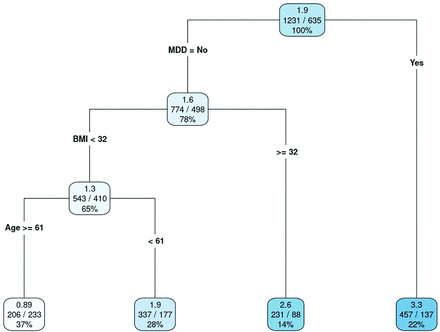
Final regression tree model for the number of overall patient burdens. Values in each leaf are as follows: top value, mean number of burdens; center values, total number of burdens reported/total number of patients (observations); and bottom value, percentage of all observations. Abbreviations: BMI, body mass index; MDD, major depressive disorder. (Used with permission of Mayo Foundation for Medical Education and Research)
A regression tree has a single tuning parameter called the complexity parameter, which controls the number of splits (also called nodes or branches) in the tree. As the complexity parameter increases, the size of the tree decreases. To choose the optimal complexity parameter and provide an estimate of model performance, we used a technique called repeated cross-validation, with 5 folds repeated 5 times.7
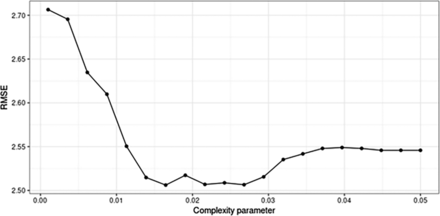
We quantified the model performance using the root-mean-squared error (RMSE), which we wanted to minimize. Appendix Figure 3 shows the mean RMSE obtained from repeated cross-validation across a range of values for the complexity parameter from 0.001 to 0.05. The mean RMSE was minimized when the complexity parameter was approximately 0.016, which yielded a mean RMSE of 2.51.
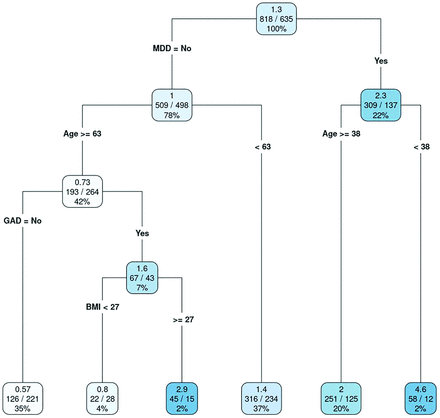
Final regression tree model for the number of personal burdens. Values in each leaf are as follows: top value, mean number of burdens; center values, total number of burdens reported/total number of patients (observations); and bottom value, percentage of all observations. Abbreviations: BMI, body mass index; GAD, generalized anxiety disorder; MDD, major depressive disorder. (Used with permission of Mayo Foundation for Medical Education and Research)
Results
Six hundred thirty-five patients completed the ICAN Discussion Aid at least once. Of those, 211 patients completed it more than 1 time, with 146 completing it twice and 65 completing it between 3 and 10 times. On average, each patient completed ICAN 1.5 times. When a patient completed ICAN more than once, the most recently completed ICAN was used for final analysis. Eighty-four ICANs were removed from analysis because they were completely blank. Table 1 summarizes the patients’ demographic and clinical characteristics: comorbid conditions and medication use associated with chronic conditions.
Overall, the number of burdens ranged from 0 to 14.0, with a median (interquartile range [IQR]) of 1.0 (0 to 3.0). The number of health care burdens ranged from 0 to 7.0, with a median (IQR) of 0 (0 to 1.0), and the number of personal burdens ranged from 0 to 10.0, with a median (IQR) of 0 (0 to 2.0). Table 2 summarizes the patients’ self-assessed burdens as reported on the ICAN Discussion Aid. The most burdensome items were memory or attention (16.4%), being active (16.1%), getting enough sleep (15.8%), emotional life (15.6%), managing diet and exercise (15.4%), work or finances (15.2%), and “the food I eat” (15.0%).
Patient Self-Assessed Burdens Using the ICAN Discussion Aid
Overall Burdens
Figure 2 shows the regression tree for overall burdens, where the criterion for each split is labeled. For each leaf, the following information is provided (from top to bottom): (1) the mean number of burdens, (2) the total number of burdens reported/the total number of patients (observations), and (3) the percentage of all observations in a given leaf. For example, 137 patients with major depressive disorder (MDD) had a mean of 3.3 overall burdens. Alternatively, 233 patients who did not have MDD and who were 61 years or older with a body mass index (BMI), calculated as weight in kilograms divided by height in meters squared (BMI), of less than 32 had a mean of 0.89 overall burdens.
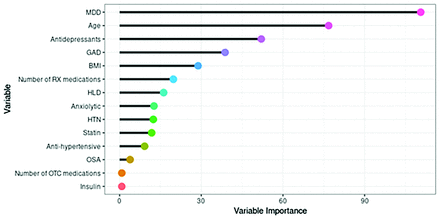
The variable importance is shown in Appendix Figure 4, where a large score indicated that the variable was important. Other candidate variables that performed similarly were identified to be used in the model in the event of missing data, which also contributed to the variable importance measure. Therefore, it was possible for a variable to be somewhat important and yet not included as a primary split in the regression tree. The following variables were important but did not contribute to the overall model: antidepressant use, hyperlipidemia, number of prescription drugs, hypertension, statin use, number of over-the-counter drugs, and insulin use.
Number of Burdens in Personal Life
We repeated the model fitting process for the number of burdens in the patient’s personal life. Repeated cross-validation identified the optimal complexity parameter as 0.014 with a mean RMSE of 1.80. In the final regression tree, MDD, age, generalized anxiety disorder, and BMI contributed to the overall model (Figure 3). As with overall burdens, some variables were considered important but did not contribute to the overall model. They are listed in order of decreasing importance here and in Appendix Figure 5: antidepressant use, number of prescription medications, hyperlipidemia, anxiolytic use, hypertension, statin use, antihypertensive use, obstructive sleep apnea, number of over-the-counter medications, and insulin use.
Number of Health Care Burdens
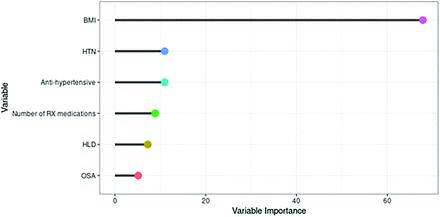
Finally, we fit a regression tree for the number of health care–related burdens. A complexity parameter of 0.037 was chosen by using repeated cross-validation with a mean RMSE of 1.12. In the final regression tree, BMI was the only variable that contributed to our model; patients with a BMI of 26 or greater had a mean burden of 0.89, and those with a BMI below 26 had a mean burden of 0.37 (Appendix Figure 6). Hypertension, antihypertensive use, number of prescription medications, hyperlipidemia, and obstructive sleep apnea also were important but did not contribute to the overall model (Appendix Figure 7).
Discussion
In this retrospective health record review of patients using the ICAN Discussion Aid, some clinical profiles were more highly associated with burden than others. Our model showed that patients already diagnosed with MDD had the highest number of overall burdens, at a mean of 3.3, compared with the mean of 1.6 total burdens in our patients without depression. Likewise, these patients had the most personal burdens, averaging 2.3 versus 1.0 in those without depression. Moreover, those patients who had major depression and were younger than 38 years had a mean of 4.6 personal burdens, 3.5 times greater than for our entire sample (1.3). This subgroup composed only 2% of our overall sample.
A much lower number of personal burdens was observed for patients without depression or generalized anxiety disorder over the age of 63 years (mean, 0.57). This cohort comprised 35% of our patients sampled. Higher BMI was highly predictive of health care burdens, with patients whose BMI was 26 or greater having a mean of 0.89 burdens and patients with a BMI less than 26 having 0.37 health care–related burdens.
These findings are important because in resource-constrained settings clinical leadership often must create decision rules to select patients who are most likely to need ICAN-based conversations and must then concentrate their implementation efforts toward the selected subgroup. To date, those decisions were based on best guesses rather than evidence of who those patients might be.
These findings are congruent with the practice paradigm of minimally disruptive medicine,3 under which ICAN was developed. In minimally disruptive medicine, patients have the baseline capacity to perform work—the demands of life, which include the burdens of prescribed medical treatment. This framework acknowledges that capacity is weakened by sociological and psychological challenges, which include illness burdens.
The ultimate goal of ICAN is to ensure that patients have health care that fits their life. Consequently, the solutions that patients and clinicians arrive at will vary greatly, with some patients perhaps needing multidisciplinary approaches to mental health care, others requiring a referral to a social worker, and still others requiring a medication review. As such, we still endorse the idea that patients should have the opportunity to discuss their life using the discussion aid with their clinician. However, we recognize through real-world implementation that resource-limited settings often are forced to select the population they think will most likely benefit. Future research should be conducted on a larger, multiclinician, and multisite cohort of patients who are administered ICAN to allow validation and modification of the present limited study. Such study would be best facilitated by incorporating the discussion aid into the EHR because our current study relied on labor-intensive manual entry of ICAN responses for analysis. ICAN was built into the EHR (Epic, Epic Systems) at our institution in April 2022, a change that will enable such future studies.
Limitations
This study has some limitations. The generalizability of these results is limited because it is a single-center study of patients cared for by a single primary care clinician. To gain further confidence in the validity and applicability of the predictors identified in this study, we must study these predictors in other data sets and across diverse settings in which ICAN is deployed within patient care. In addition, we used the most recent data at EHR review as a best estimate of a patient’s clinical characteristics when they completed the ICAN Discussion Aid. This estimate was necessary for uniformity and convenience but was not completely accurate, especially because treatment plan alterations are expected to occur because of the ICAN discussion. This limitation was most notable when we considered cancer diagnoses, which may have had a volatile yet important impact on our patients and that we may have captured inadequately. Our regression trees created a straightforward interpretation of our results, but we acknowledge that a more powerful predictive algorithm such as a gradient-boosted machine likely would have produced a more accurate model, albeit at the cost of interpretability. Finally, our study focused on chronic diseases of interest to a medical clinician as opposed to a standard list of accepted chronic conditions, and this choice could have introduced unintentional bias into the study.
Conclusion
We attempted to create a model that predicts which patients will report a high number of burdens on the ICAN Discussion Aid, and our findings illustrate that such a model would be useful for the judicious study and application of ICAN. In our patient population, certain demographic and clinical characteristics were associated with more burdens, especially the characteristics of younger age, high BMI, and a depression diagnosis. As the use of ICAN becomes more focused, clinicians may have a better chance of partnering with highly burdened patients to make care fit.8 Greater investigation of ICAN is necessary. The future goal should be to flag at-risk patients so that more in-depth conversations can take place to form well-fitting programs that maximally support patient goals while minimally disrupting their lives and loves.
Acknowledgments
Kathleen Louden, ELS, senior scientific/medical editor, Mayo Clinic, substantively edited the manuscript. The Scientific Publications staff, Mayo Clinic, provided proofreading, administrative, and clerical support.
Appendix
Instrument for Patient Capacity Assessment (ICAN) discussion aid. Outside bifold of flyer lists open-ended questions. (Used with permission of Mayo Foundation for Medical Education and Research)
Histogram of number of patient burdens. (Used with permission of Mayo Foundation for Medical Education and Research)
Average Root Mean Squared Error (RMSE) across the complexity parameter for the total number of burdens. (Used with permission of Mayo Foundation for Medical Education and Research)
Variable importance plot for the regression tree modeling the number of overall burdens. Abbreviations: BMI indicates body mass index; HLD, hyperlipidemia; HTN, hypertension; MDD, major depressive disorder; OTC, over-the-counter; RX, prescription. (Used with permission of Mayo Foundation for Medical Education and Research)
Variable importance plot for the regression tree modeling the number of burdens in the patient’s personal life. Abbreviations: BMI indicates body mass index; GAD, generalized anxiety disorder; HLD, hyperlipidemia; HTN, hypertension; MDD, major depressive disorder; OSA, obstructive sleep apnea; OTC, over-the-counter; RX, prescription. (Used with permission of Mayo Foundation for Medical Education and Research)
Final regression tree model for the number of health care burdens. Values in each leaf are as follows: Top row, Mean No. of burdens; Center, Total No. of burdens reported/total No. of observations (patients); and Bottom, Percentage of all observations. Abbreviations: BMI indicates body mass index. (Used with permission of Mayo Foundation for Medical Education and Research)
Variable importance plot for the regression tree modeling the number of health care burdens. Abbreviations: BMI indicates body mass index; HLD, hyperlipidemia; HTN, hypertension; OSA, obstructive sleep apnea; RX, prescription. (Used with permission of Mayo Foundation for Medical Education and Research)
Notes
This article was externally peer reviewed.
Funding: Dr. Boehmer was supported as a Minnesota Learning Health System (MN-LHS) Scholar by the Agency for Healthcare Research and Quality (AHRQ) and Patient-Centered Outcomes Research Institute (PCORI) (grants K12HS026379 and T32HS026379) and by the National Institutes of Health’s National Center for Advancing Translational Sciences (grant KL2TR002492). Additional support for MN-LHS scholars is offered by the University of Minnesota Office of Academic Clinical Affairs and the Division of Health Policy and Management, University of Minnesota School of Public Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ, PCORI, or MN-LHS Mentored Career Development Program. The funder had no involvement in the study design; data collection, analysis, and interpretation; writing of the report; or the decision to submit the article for publication.
Conflict of interest: The authors report no conflicts of interest.
To see this article online, please go to: http://jabfm.org/content/36/2/277.full.
- Received for publication July 22, 2022.
- Revision received October 27, 2022.
- Accepted for publication November 1, 2022.