Abstract
Background: The opioid crisis presents many challenges for family practice providers in rural communities who treat patients with chronic non-cancer pain (CNCP). Unfortunately, evidence for effective opioid reduction strategies is sparse. We evaluated the effects of implementing a comprehensive opioid reduction protocol on overall opioid prescribing among patients with chronic non-cancer pain in our rural family medicine clinics.
Methods: We compared mean daily milligrams morphine equivalent (MME) prescribed to patients with CNCP in our rural family medicine clinic (n = 93) with another matched clinic (n =93) after implementation of our comprehensive protocol. We also compared mean daily MME prescribed to our patients with CNCP before and after implementation of the protocol. In a subsequent cross over phase, we examined the effects of the protocol when applied to the original control group patients.
Results: Mean daily MME in the intervention clinic (29.77) was significantly lower than the control clinic (93.2) after the intervention (t = 6.03; P < .00). Mean daily MME in the intervention group was significantly lower after implementation of the protocol (29.77) than before the protocol (MME 80.34) (t = 5.889; P < .00). After crossover, the mean daily MME was significantly lower (14.34) in the original control group than prior to the cross over intervention (85.68); (t = 8.19; P = .00).
Discussion: Our comprehensive opioid reduction protocol led to significant reductions in opioid prescribing in our rural family medicine clinics. Future studies should include important qualitative outcome measures such as patient function.
- Chronic Pain
- Cross-Over Studies
- Opioid Epidemic
- Opioid-Related Disorders
- Opioids
- Outcomes Assessment
- Physician's Practice Patterns
- Rural Health
Background
Significant decreases in opioid prescribing occurred after the 2016 release of the US Centers for Disease Control and Prevention (CDC) Guidelines for Prescribing Opioids for Chronic Pain.1,2 Nevertheless, the opioid crisis continues to present many challenges for family practice providers in rural communities, who treat patient with chronic noncancer pain (CNCP) on chronic opioid therapy (COT). In 2017, 47,600 drug overdose deaths (67.8%) involved an opioid.3 Of the 15 counties with the highest opioid prescribing rates, 14 of those are rural.1 Patients from the most rural settings had an 87% higher chance of receiving opioid prescriptions compared with urban populations.1 Recent efforts at reducing prescription opioids may have contributed to an increase in heroin use among patients with opioid use disorder (OUD).4 Some providers went beyond CDC guidelines, abruptly stopping all opioids or transferring all their CNCP patients, prompting the CDC to advise against misapplication of the guidelines.5 In 1 state, half of patients on at least 120 milligrams morphine equivalent (MME) had no dose reduction before opioid discontinuation. Forty-nine percent of these patients experienced adverse events such as emergency department visits or hospital admission due to opioid poisoning or substance use disorder.6 Complete discontinuation of opioids among patients with CNCP may actually increase the risk of overdose death7 and every additional week of tapering time was associated with a 7% reduction in adverse events.6
Adding to the challenge of treating patients with CNCP is that the condition itself may not be a single, homogeneous entity. Chronic pain usually involves a physiologic “pain generator” such as osteoarthritis of the cervical or lumbar spine with nerve root impingement and radiculopathy. However, many CNCP patients also have significant overlays of comorbid psychiatric illness, substance use disorders, or both.8⇓–10 In addition patients with past psychiatric trauma may experience real chronic pain via a psychophysiological mechanism.11 Combinations of these factors can lead to various chronic pain “phenotypes”12 making diagnosis and treatment more challenging.
In light of these challenges, family practice providers in rural setting are left with the daunting task of reducing opioid medications to levels recommended by the CDC, often with limited community resources, such as behavioral health, pain medicine specialists, and medication-assisted therapy (MAT). Unfortunately, evidence on effective opioid reduction strategies is sparse. A quality improvement intervention, including prescribing registries, a nurse coordinator, and an opioid use review panel demonstrated a 22% reduction in opioid prescribing using a noncontrolled, before and after design.13 In a 2017 Cochrane review, the authors found only 5 randomized controlled trials addressing interventions to reduce opioids in CNCP. The trials were too heterogeneous to conduct a meta-analysis. Only 3 of the 5 studies actually reported opioid dose as the outcome variable and these trials were small (n = 35 to 55).14 Electro-acupuncture showed promise in opioid reduction (P = .056) but the effect did not last at follow up and the study was compromised by a high dropout rate.15 An opioid taper support protocol (psychiatric consultation, opioid tapering, and a weekly cognitive behavioral therapy [CBT] with a physician assistant), while helping with pain interference and self efficacy, did not lead to a significant decrease in daily MME compared with treatment as usual.16 After an 11-week CBT group, patients who received a weekly automated, therapeutic telephone call had a significant decrease in opioid use compared with controls.17 Another 2017 “review of reviews” found a lack of high-quality evidence or consistent findings on optimal treatment approaches for patient with co-occurring CNCP and substance use disorder.18
The purpose of our study was to evaluate the effects of implementing a comprehensive opioid reduction protocol on overall opioid prescribing among patients with chronic noncancer pain in our rural family medicine clinic. Our comprehensive opioid reduction protocol incorporated several of the CDC 2016 opioid prescribing guidelines.2 Our research question was: among patients with chronic, noncancer pain, what effects, if any, will implementing a comprehensive opioid reduction protocol have on overall opioid prescribing in our rural family medicine clinic?
Methods
We compared overall opioid prescribing in mean daily MME before and after implementation of a comprehensive opioid reduction protocol among our clinic patients with CNCP (with-in group analysis). We also compared mean daily MME between our clinic and a matched rural family medicine clinic before and after implementation of the protocol (between groups analysis). Our prospective cohort design also included a cross over phase. In the initial intervention phase, the protocol was applied only to the intervention group. In the subsequent cross over phase, the protocol was then applied to the original control group patients.
Our study population was adult patients with CNCP on COT from 2 rural family medicine clinics. Chronic pain was defined as pain that lasted longer than 3 months or beyond the expected time of healing.19 COT was defined as the use of opioid medications on most days for longer than 3 months.20
Inclusion criteria were adult patients (male and female) diagnosed with CNCP on COT. Exclusion criteria were patients with a cancer diagnosis (other than basal and squamous cell), pregnancy, and patients receiving MAT (buprenorphine or methadone clinic) for an OUD.
Study participants (n = 186) were a convenience sample drawn from our clinic's COT patients (n = 93) and those from a nearby matched clinic (n = 93). Participants were not randomized to intervention or control groups. Rather, 1 clinic was designated as the intervention cohort and the other, the control or “treatment as usual” cohort. The similarities between these 2 clinics included the following: same county, same health system, same electronic medical record; both had rural health clinic designation, both were primary care family medicine clinics, both were in small rural communities, and providers at each clinic had similar credentials (family physician and physician assistant).
Each clinic's COT population was stratified based on levels of MME. Patients in each stratum were selected randomly in equal numbers so that each stratum was matched between the intervention and control group.
Our independent (predictor) variable was our opioid reduction protocol. (Table 1) Our primary dependent (outcome) variable was mean daily MME. Secondary outcome variables included: number of patients off COT, number of patients transferred to pain specialists, number of patients who dropped out of care (no primary care provider and not managed by pain specialists), and number of deaths from any cause during intervention.
Components of the Comprehensive Opioid Reduction Protocol for Rural Chronic Non-Cancer Pain Patients: Original Intervention and the Crossover Arm (n = 186)
Our opioid reduction protocol consisted of several interventions primarily guided by the CDC guidelines: risk assessment and mitigation; patient education via an 8-week psychoeducational group (Table 2); checking urine drug screens and state-wide prescription drug monitoring program; treating psychiatric comorbidities; maximizing nonpharmacological and nonopioid pharmacological treatments; slow taper of opioids at 10% of original dose per month, aiming to get all patients under 90 MME and most under 50 MME; and finally assessment for OUD with referral for MAT if indicated.
Description of Psycho-Educational Group for Rural Chronic Non-Cancer Pain Patients
Our clinics underwent changes between the original and the crossover intervention, which necessitated slight alteration in the protocol. The group facilitators became busy with other responsibilities and did not have time to run groups. In addition, the clinics gained new leaders who were more risk adverse to COT in CNCP patients. Due to these changes, the protocol was slightly varied during the cross over phase including: patient education was provided by individual providers and patient handouts rather than group education; more high risk patients were referred to pain specialists; we aimed for a lower level of MME (goal of 30 MME) or to get patients off opioids entirely and there was slightly faster intervention time of 1 year rather than 2 years (Table 1).
Statistical analysis was performed by Statistical Package for Social Sciences (SPSS), version 25 (IBM, Armonk, NY). Nominal data were analyzed with chi-squared or Fisher's Exact tests. Ordinal data were analyzed with Wilcoxon–Man-Whitney U test. Continuous with-in group data were analyzed using dependent sample t-test. Continuous between groups data were analyzed using independent sample t-test. A priori significant level was set at α = 0.05 and sample size estimate was 148. Neither participants nor clinicians were blinded. Patients who were diagnosed with OUD and receiving MAT (5.9%) as well as patients who were diagnosed with cancer (1.08%) during the study were excluded from analysis. Otherwise, the intention-to-treat principle was followed. Our study was approved by our health care system's institutional review board.
Results
Our intervention and control groups were similar convenience samples, but they were not randomized, thus losing the equal distribution effects that randomization affords. As a result, the intervention and control group were similar on many, but not all demographics. Groups did differ in the following demographics: more patients in the control group were diagnosed with anxiety disorders and on benzodiazepines; more patients in the intervention group had histories of smoking, substance abuse, and cervical/lumbar radiculopathy (Table 3).
List of Demographics for Our Rural Chronic Non-Cancer Pain Patients: Differences Between the Intervention and Original Control Group before Intervention (n = 186)
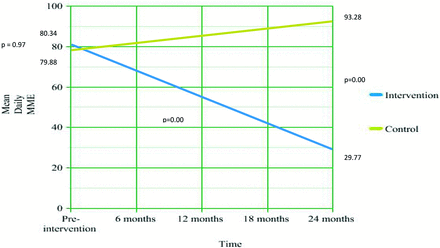
Mean daily MME in the control clinic did not change significantly during the initial intervention period (MME, 79.88 before; MME, 93.28 after; t = 1.6; P = .11). Mean daily MME in the intervention group was significantly lower after implementation of the protocol (29.77) than before the protocol (MME, 80.34) (t = 5.889; P < .00).
Mean daily MME was not different between the intervention (80.34) and control (79.88) groups before intervention (t = 0.04; P = .97). Mean daily MME in the intervention clinic (29.77) was significantly lower than the control clinic (93.2) after the protocol (t = 6.03; P < .00) (Figure 1).
Change in opioid mean daily Morphine Milligram Equivalents (MME) among rural chronic non-cancer pain patients over time (n = 186).
After the study protocol was applied to the control group in the cross over phase, the mean daily MME was significantly lower (14.34) than before the crossover intervention (85.68); (t = 8.19; P = .00) (Figure 2).
Change in opioid mean daily Morphine Milligram Equivalents (MME) in rural chronic non-cancer pain patients during 1 year cross over phase (n = 93).
In exploring our secondary outcomes, we observed that opioids were stopped on significantly more patients in the crossover phase (53.76%) than in the original intervention (27.96) (P = .00). In addition, significantly more patients were referred to pain specialty in the crossover phase (43.01%) than in the original intervention (6.45%) (P = .00). Nevertheless, the number of patients who dropped out of care entirely from the crossover group (8.6%) was not significantly different from the original intervention group (4.3%) (P = .37). In addition, the number of patients who died from any cause during the crossover arm (7.5%) was not significantly different from in the original intervention group (4.3%) (P = .54). The percent of patients who died from any cause during the original intervention (4.3%) and crossover arms (7.5%) were lower than the annual crude death rate in our state (9.8%) (Table 4).
Differences Between Our Rural Chronic Non-Cancer Pain Groups on Secondary Outcomes
While not a formal intervention or outcome measure in our protocol, several of our clinicians asked our CNCP patients about adverse childhood experiences (ACES), including childhood physical and sexual abuse.21 Qualitatively, many of our patients with chronic pain on COT did report significant childhood loss, abuse, and trauma.
Discussion
Our comprehensive opioid reduction protocol led to significant reductions in opioid prescribing in our rural family medicine clinics. This significant reduction in opioids was demonstrated both within the intervention group and between the intervention and control groups. The efficacy of our protocol in reducing opioids was again demonstrated, when applied to the control group during crossover, further suggesting that the effects of the protocol were real.
As noted, the protocol was changed somewhat during the crossover due to new clinic leaders who were more risk adverse to COT in CNCP patients. Despite the changes, there were no significant increases in dropout rates or mortality, suggesting that tapering over 1 year, referring high-risk patients to pain specialists, and aiming for the lower opioid thresholds (30 MME or lower) may be done without increases in patient drop out or mortality.
We hypothesize that our active patient education, attention to risk reduction, treatment of psychiatric comorbidities and attentive management of pain with nonmedication and nonopioid pain medicines, all contributed to patient retention and low mortality rates. In addition, due to our slow taper (10% per month), our patients had almost no withdrawal symptoms. This likely also contributed to our low drop out and mortality rates.
Our study has several limitations. The control and intervention groups were based on convenience samples and were not randomized. This may have contributed to several differences between groups before intervention. The patients, clinicians, and researchers were not blinded, possibly leading to the Hawthorn and Observer Expectancy Effects. Our sample size, while larger than many studies in the opioid reduction literature (n = 186), was still modest. Several aspects of the protocol were modified during the crossover arm. Our pain specialty consultant was a 45-minute drive away and many of our patients had transportation issues. This may have affected their continuation in treatment, confounding our dropout rate. We did not assess several important qualitative outcomes such as pain control, patient function, and patient/provider satisfaction. Finally all components of the comprehensive protocol were implemented together, making analysis of the individual effects of each component difficult.
Our study did have several strengths. Our prospective, controlled, cohort design adds robustness to our study. Our intervention and control clinics were very similar on many demographics before intervention. We used a randomized stratified sampling method to ensure groups were matched at each stratum of MME. This led to no difference between groups on mean daily MME before intervention. Our application of the protocol to the original control group during the crossover arm helped to verify that the effects of our protocol were real and not due to chance. We prioritized fidelity to the intention-to-treat principle. Finally, because of our ability to track participant's prescription opioid use via the state wide prescription drug monitoring program, there was almost no loss to followup (1%).
This study provides a relevant contribution to the literature. We found that the CDC guidelines2 for opioid prescribing can be effectively implemented in a rural family medicine clinic. Ours may be one of the few opioid reduction studies that incorporates the CDC guideline as the centerpiece of the intervention.14 With our n of 186 patients, ours is one of the larger opioid reduction studies.14 We found that clinicians can be mindful of and responsive to patients psychiatric needs while tapering opioids.8 Most of our patients were compliant with and tolerated a 10% per month taper very well, supporting the value of a slow tapering approach.6.7
Despite these contributions, design constraints such as our convenience sampling may limit extrapolation of our results. In addition, we did not include important qualitative measures such as withdrawal symptoms, pain level and daily function as outcomes. Recommendations for improved future studies include larger samples, randomized controlled trials and inclusion of qualitative outcome measures such as pain control, patient function, patient and provider satisfaction, and economic analysis.
We encountered several barriers during the implementation of our protocol. While we have 3 outpatient substance abuse clinics in our county, we had no direct referral path to them for our substance dependent patients. We have no pain specialist in our county and the closest was a 45-minute drive. We had no waivered clinicians in our county to provide MAT for our patients diagnosed with OUD during most of the intervention period. Finally, due do the stigma sometimes associated with patient on COT, our office staff had anxiety about some of the interventions in our protocol.
To overcome these barriers, we implemented a number of strategies. Several of our clinicians personally met with substance abuse therapists in our county to build bridges and discuss how to streamline referrals. We met with our regional pain specialist on a number of occasions and developed a good relationship with him. He then provided consultation on difficult cases over the phone. Two of our clinicians (a family medicine MD and physician assistant) became waivered to prescribe and manage buprenorphine for our patients. Finally, we found that by enlisting key providers and staff in the development of our protocols, listening to staff anxieties and providing staff education with patience, we were able to obtain good team cooperation with our interventions.
Next steps include sharing our protocol and results with other family medicine clinics, hoping they will consider implementing and building on our interventions. We are striving to increase psychological services for our CNCP and OUD patients by building bridges with community behavioral health clinics. Finally we hope to work with community partners to increase mental health services to at-risk families with young children and to promote treatment options for adults who suffer the sequela of childhood trauma. Like others, we speculate that adverse childhood events and trauma may correlate with adult chronic pain and the use of opioids to anesthetize this physical and psychic pain.11,21,22 If we hope to continue our progress against the opioid crisis, we believe we need to join with community partners in treating psychological trauma in adults and preventing the abuse and neglect of our children.
Conclusion
Using a slow taper approach and implementing key CDC guidelines such as assessing and mitigating risk, providing attentive patient education, treating psychiatric comorbidities, and increasing nonopioid pain treatments, we were able to safely reduce opioid prescribing in our rural family medicine clinic. We encourage other rural family medicine clinics to confirm the effectiveness of our interventions with larger, randomized controlled trials that include important qualitative aspects of opioid reduction such a pain control, patient function, patient/provider satisfaction and economic analysis.
Acknowledgments
The authors wish to express our gratitude to Abid Khan, MD for his generous and wise guidance to clinicians and his excellent care for the patients of our communities.
Notes
This article was externally peer reviewed.
Conflicts of Interest: Each author report no conflicting or competing interests.
Funding: None.
To see this article online, please go to: http://jabfm.org/content/33/4/502.full.
- Received for publication January 31, 2020.
- Revision received April 28, 2020.
- Accepted for publication April 28, 2020.