Abstract
Background: Previous work has shown that $210 billion may be spent annually on unnecessary medical services and has identified patient and hospital characteristics associated with low value care (LVC). However, little is known about the association between primary care physician (PCP) characteristics and LVC spending. The objective of this study was to assess this association.
Methods: We performed a retrospective analysis by using Medicare claims data to identify LVC and American Medical Association Masterfile data for PCP characteristics. We included PCPs of adults aged 65 years and older who were enrolled in Medicare in 2011. We measured Medicare spending per attributed patient on 8 low value services.
Results: Our final sample contained 6,873 PCPs with 1,078,840 attributed patients. Lower per-patient LVC Medicare spending was associated with the following PCP characteristics: allopathic training, smaller Medicare patient panel, practiced family medicine, practiced in the Midwest region, were a recent graduate, or practiced in rural areas. The largest associations were seen in Medicare patient panel size and geographic region. The average per-patient LVC spending was $14.67. LVC spending among PCPs with small patient panels was $3.98 less per patient relative to those with larger panels. PCPs in the Midwest had $2.80 less per patient LVC spending than those in the Northeast.
Conclusion: Our analysis suggests that LVC services are associated with specific PCP characteristics. Further research should assess the strength of these associations, and future policy efforts should focus on systemic interventions to reduce LVC spending.
Total health care spending in 2015 reached $3.2 trillion and comprised 17.8% of the gross domestic product.1 One concern is that a significant proportion of this spending is in unnecessary care. The authors of a National Academy of Medicine report defined unnecessary services as “overuse—beyond evidence-established levels, discretionary use beyond benchmarks; [and] unnecessary choice of higher-cost services,” and found that $210 billion per year is spent on unnecessary services.2 Another study found that 30% of all Medicare spending is unnecessary.3
Studies have attempted to characterize patient and regional characteristics associated with unnecessary services, often referred to as low value care (LVC). One study compared LVC services received in Medicaid and commercial insurance patients finding 14.9% of Medicaid patients and 11.4% of commercial insurance patients received at least 1 LVC service in the year. There was no association between insurance type and likelihood of LVC.4 A study looking specifically at safety net populations showed no difference in LVC based on insurance type or based on whether the patient was seen at a safety-net clinic or by non-safety-net physicians.5 Others have shown hospital-based practices and areas with a higher specialist to primary care physician (PCP) ratio are associated with more LVC.6,7 Mafi et al.7 found significantly more unnecessary computer tomography and magnetic resonance imaging (8.3% vs 6.3%) and specialty referrals (19% vs 7.6%) in hospital-owned practices than in physician-owned practices.
Identifying LVC events within claims data has proven difficult. Studies select specific LVC services based on generally accepted guidelines, such as Choosing Wisely and the United States Preventive Services Task Force.8⇓⇓–11 For each LVC service, there are situations in which receiving the service would not be deemed low value. For example, back imaging is not considered low value in a patient with known cancer. Schwartz et al.12 defined claims based measures of LVC by looking at a more specific and more sensitive version of each LVC service. As expected, they found more beneficiaries receiving LVC services when using the more sensitive measure.12 It is also difficult to compare LVC studies because there is variability in the measure of LVC depending on the service measured, how it is being measured, and the population for which it is being measured. Colla et al.6 showed a wide range of annual prevalence from 1.2% to 46.5% depending on which LVC service is measured. The population is another concern, as different studies have used different data sets, including Medicare data, Medicaid data, and commercial insurance data. The LVC use patterns and necessary interventions in, for example, the Medicare population may be different from the commercially insured population.
Despite the complexity of measurement, it is important to assess and understand LVC services particularly in this setting of increasingly greater health care costs.1 Past studies have identified regional and patient characteristics and clinic settings associated with LVC, but no study to our knowledge has focused on individual physician characteristics.5 In this study, we aimed to assess the characteristics of physicians who have patients with lower LVC spending.
Methods
Data
We considered primary care services that were deemed low value by the Choosing Wisely campaign, relevant to the elderly population and defined in previous studies.6,12 We used 4 different data sources in our analysis: 2011 Medicare Claims Carrier File to identify LVC services and spending, physician specialty, practice location, panel size, and patient panel characteristics; 2010 American Medical Association Masterfile for additional physician characteristics; 2008 to 2012 American Community Survey (ACS) 5-year Zip Code Tabulation Area (ZCTA)-level estimates for physician location characteristics; and 2013 United States Department of Agriculture Rural-Urban Continuum Codes (RUCC) file to identify rural areas.13
Study Sample
We had a nationally representative, stratified random sample of 38,516 physicians. We restricted the sample to PCPs defined as physicians specializing in family medicine, internal medicine, general practice, and geriatric medicine, ending up with 6,873 PCPs. We matched these PCPs with Medicare beneficiaries who had received the plurality of Part B services from them. We restricted beneficiaries to those who were aged 65 and older, without end-stage renal disease, covered by both Part A and Part B for the entire 12-month period from Jan 2011 to Dec 2011 unless death was the cause of discontinuation, and were not a member of a Health Maintenance Organization (including Medicare Advantage) in any given month. We omitted patients and PCPs with missing values and PCPs who had less than 20 attributed patients (See Appendix for details).
LVC Services And Outcome Variables
Previous studies had developed Medicare claim-based measures of LVC services.6,12 We used these measures to identify LVC services that are relevant to primary care, relevant to patients 65 years and older, and measurable using Medicare claims data. There were 8 LVC services that met these criteria: low back pain imaging in the first 6 weeks of diagnosis, brain imaging for simple syncope, screening for osteoporosis in men younger than 70 years, cardiac screening for low-risk asymptomatic patients, prostate cancer screening, routine preoperative testing for low-risk surgical procedures, carotid artery disease screening, and cervical cancer screening for women aged 65 and older. For each LVC service type, we first identified qualifying patients who were eligible for the service based on diagnoses, demographics, and other criteria from all patients that had received the service. We then excluded those patients whose receipt of service could be “justified” based on their medical history, as reported in the carrier claims, before the service date. Therefore, for each LVC service type, the services provided to type-specific qualifying patients that did not satisfy the exclusion criteria were deemed LVC (see Appendix Table 1).
Our main outcome variable was physician-level LVC Medicare spending per-attributed-patient ($), calculated by summing up Medicare payments across the above 8 LVC services received by their attributed patients and then dividing by the number of attributed patients. We also assigned PCPs into 2 groups—a high and low LVC group—with the high LVC group being those whose per-attributed-patient LVC spending was in the top quintile and the low LVC group being all other PCPs.
Physician Characteristics
PCP characteristics of interest included sex, years in practice, specialty, credential type, international medical graduate status, patient panel size, and practice location in terms of region and rurality. Rurality was defined as a nonmetropolitan county indicated by the RUCC.14
Analysis
We explored whether specific PCP characteristics were correlated with observed variation in their attributed Medicare patients' overall LVC spending by using an ordinary least squares (OLS) regression model. We adjusted our model for demographic characteristics and health status of the PCP's patient panel by including its age, race/ethnicity, sex distributions, and health status distributions, as measured by an Elixhauser comorbidity “index.”15 We also compared the adjusted odds of having high per-attributed-patient LVC spending across PCPs' physician characteristics by using a logit model. All regression models were weighted to take into account the oversampling of physicians in relatively smaller states. Several sensitivity analyses were performed to determine the robustness of our findings (see Appendix for details).
Results
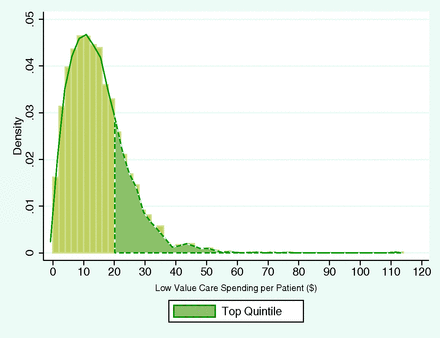
Our final sample contained 6,873 PCPs with 1,078,840 attributed patients. Although the average share of LVC spending in total noninstitutional Part B Medicare spending of the attributed patient panel was 0.5%, there was significant variation in per-patient LVC spending across PCPs (Figure 1). The sample distribution was skewed to the right, indicating the presence of a small number of PCPs with “relatively extreme” LVC spending. The physician LVC spending-per-patient ranged from $0 to $112.62, with mean spending at $14.67. The threshold value for top quintile was $18.86. All dollar amounts are in 2011 dollars.
The Primary Care Physician Sample Distribution of per-patient Low Value Care Medicare Spending ($).
Table 1 shows that relative to the high LVC group, the PCPs in the low LVC group were on average more likely to be female (29% vs 23%), practice family medicine (52% vs 39%), and have a smaller Medicare patient panel (147 vs 189 patients). They were also on average more likely to practice in the Midwest (33% vs 12%) and rural areas (20% vs 6%). They were more like to have an older, healthier, and more female patient population. There were no discernible differences in the average racial/ethnic composition of patient panel between the 2 groups. All above differences were statistically significant (P < .001). (Table 1).
Comparison of Characteristics between Low and High Low Value Care Spending Groups Among Primary Care
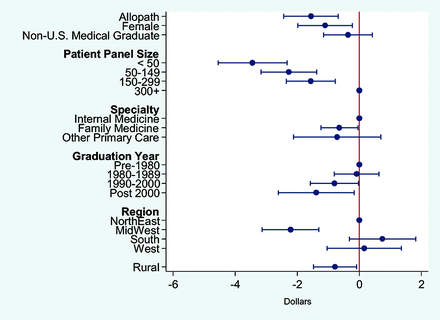
After adjusting for patient and practice location characteristics, lower per-patient LVC Medicare spending was associated with the following PCP characteristics: allopathic training, smaller Medicare patient panel, practiced family medicine, practiced in the Midwest region, were a recent graduate, or practiced in rural areas.. PCPs who were allopathic-trained physicians had, on average, lower per LVC spending relative to osteopathic PCPs by $1.65. PCPs who had less than 50 attributed Medicare patients and between 50 to 149 attributed patients had, on average, $3.98 and $2.73, respectively, lower annual LVC spending relative to those PCPs who had more than 300 attributed Medicare patients. Those who practiced family medicine had on average $1.03 lower LVC spending than those who practiced internal medicine. The adjusted mean differences in per-patient LVC spending between those PCPs that practiced in the Midwest and those that practiced in the Northeast Region was $2.80, whereas the difference between the rural and urban PCPs was −$1.75. The weighted mean per-patient LVC spending was $14.67. (Figure 2 and Appendix Table 2) Logit regression results were consistent with the above findings (Appendix Figure 5 and Appendix Table 3).
Association between Physician Characteristics and per-patient Low Value Care Medicare Spending ($).
Discussion
To our knowledge, this is the first study to describe LVC spending at the individual physician level.6,12 We identified physician characteristics associated with patients who had lower LVC spending. These characteristics included an allopathic medical degree, family medicine specialty, practicing in the Midwest and in rural areas, and having a relatively smaller Medicare patient panel.
Our findings showed that rurality and PCP specialty were strong predictors of per-patient LVC Medicare spending. However, given that relatively more family physicians practiced in rural areas, it was not clear whether rurality and specialty were independent predictors. We added interaction terms in our regression models to explore whether the observed differential in LVC spending by rurality in our findings was due to differences in the composition of PCP specialty between rural and urban areas. The standard errors of our estimates were too large to answer this question, but the coefficient estimates of rurality and family medicine were robust with its size increased, implying that rurality and physician specialty were independently associated with LVC spending (see Appendix Table 3).
Rurality was independently associated with less LVC. Consistent with the findings of Starfield et al.16 documenting primary care's cost savings, Colla et al.6 found that hospital referral regions with lower specialist to primary care ratios also use less LVC.6,16 Our study did not include specialists in our sample, so it remains unclear if there is something unique about the physicians and patients living in rural areas that leads to reduced use of LVC or if the decreased presence of specialists leads to reduced use of LVC.
We used a model of attributing patients to the PCP who provided the most care. Although this limits what we can say about the individual PCP, it may add to the evidence for the role of primary care in reducing costs related to LVC (or in reducing unnecessary care). Previous studies have shown that hospital-based practices and areas with higher specialist to PCP ratios have higher LVC spending.6,7 Perhaps family doctors in rural areas in the Midwest are more likely to have stronger patient attribution, involve fewer specialists, and, therefore, have less LVC spending. Through this method of patient attribution, perhaps this study adds to the evidence for the value of primary care in providing comprehensive, high value, evidence-based care.
These findings could inform a model of health system characteristics that lead to LVC. Previous studies have shown urban physicians tend to have a narrower scope of practice, more episodic care, and higher referral rates than their rural counterparts.17,18 Family physicians with a broader scope of practice have been associated with significantly lower Medicare costs.19 We found the total noninstitutional Part B Medicare spending per patient was higher in those physicians with attributed patients that have higher LVC spending. Perhaps a broader scope of practice (like that characteristic of rural physicians) may lead to a decrease in LVC services, although this will need to be directly measured in future studies.
In addition, there is an increasing tension between volume and value in health care.20,21 This study found that physicians are more likely to be in the high LVC group when they have more attributed Medicare patients. Alhough it is not possible to determine with Medicare claims data alone, future research should seek to find if a larger total patient panel is associated with higher LVC spending. It is possible that those with smaller panels may be able to minimize LVC by engaging patients in shared decision making or that patients who use less LVC are more likely to choose a PCP with a smaller patient panel. Unfortunately, our data set and methods did not allow us to elucidate the specific mechanisms. More detailed analysis of patient panel size and LVC could help to inform patient empanelment discussions.
There have been some efforts to reduce LVC on a local level with variable results.22,23 It is possible that system interventions, such as working to support a broader scope of practice, might be another approach to reduce LVC. Future studies should both continue to look at local interventions and focus on systemic efforts to decrease the use of LVC services.
Limitations
Several limitations are noted. First, whether our attribution approach was representative of the true PCP patient panel cannot be tested. If the attributed PCP-patient relationship was weak, meaning patients often see a physician who is not their PCP the most times, our findings may have been driven by unobserved patient characteristics. If the attribution strength was correlated with physician characteristics and if those patients with weaker ties to their PCPs were more likely to seek LVC services, then the estimated associations would be biased. Second, we have limited information on patients, including their socioeconomic status. This means we cannot rule out the possibility that the observed associations between physician characteristics and LVC spending were driven by unobserved patient panel characteristics. Third, our study only focused on LVC costs to Medicare and did not directly address the rate of LVC use. Fourth, our exclusion criteria for LVC services were based on medical history recorded in claims before, but during the same year as the service date, due to data availability. We were not able to observe diagnoses that occurred before 2011, which may have led to an overcounting of LVC services. As long as the magnitude of overcounting did not differ systematically across physician characteristics, our estimates were unaffected. Fifth, we were limited to LVC services that can be measured in Medicare claims data and those that occurred in a noninstitutional setting, excluding PCPs who work primarily in safety-net institutions. Finally, the generalizability of our results is limited to the elderly population.
Continued research into LVC services is important, as we focus on value in health care. We found that the average LVC Medicare spending in our sample was 0.5% of total spending with a wide range. Previous reports have estimated that 30% of Medicare spending is on unnecessary or redundant care.3 There are multiple possible contributors to this difference between our finding and previous estimates, including that we did not measure all LVC services because of limitations of secondary data analysis, did not include LVC services provided in nonoffice (eg, clinics and hospital outpatient departments)-based settings, and did not include redundant care in our analysis. A possible contributor to this difference is that our sample excluded specialists. Perhaps patients who obtain all their care from specialists are more likely to have higher LVC spending than patients who see a PCP for part of their care.
Conclusion
An estimated $210 billion dollars are spent each year on unnecessary services.2 We found that physician characteristics associated with having attributed Medicare patients who had lower LVC Medicare spending included living in the Midwest, practicing in a rural area, having an allopathic medical degree, having a family medicine specialty, and having a relatively smaller Medicare patient population. Future studies should further characterize physicians with fewer LVC services and might consider other potential factors, such as scope of practice and role of primary care. By understanding characteristics associated with less LVC, we can begin to strategize on ways to decrease medical waste in the United States.
Appendices
Data Sources
We used the 2011 Medicare Claims Carrier File to attribute Medicare beneficiaries to PCPs and to obtain information on physician specialty, practice location, panel size, and beneficiary-level characteristics and LVC spending. The rurality of practice location was determined using the 2013 United States Department of Agriculture's county-based RUCC, which divided counties into 3 metropolitan and 6 nonmetropolitan county categories. Other demographic and biographic physician information, namely, age, sex, medical school graduation year, specialty, credential type, and international medical graduate status, was extracted from the 2010 American Medical Association's Professional Physician Data of the Masterfile. Physician location community characteristics were obtained from the American Community Survey 5-year ZCTA-level estimates for 2008 to 2012. The 2011 Uniform Data System Mapper's Zip code to ZCTA crosswalk was used to link 5-digit practice location ZIP code in the claims data to its ZCTA.
Sample Construction
We began with a stratified nationally representative random sample of 38,516 physicians from the 2010 American Medical Association Masterfile and their 2011 Medicare carrier claims. Because we were only interested in PCPs, we identified those physicians whose most frequently reported provider specialty in the Carrier (Part B) line file were family practice, internal medicine, general practice, or geriatric medicine. This limited our sample to 9,904 PCPs. We then matched these PCPs with Medicare beneficiaries, who had received the plurality of Part B primary care services, as reported in the carrier line file, from them in a noninpatient setting during the year 2011. We defined primary care service as a medical service provided by a PCP. We restricted Medicare beneficiaries to those who were aged 65 and older, without end-stage renal disease, covered by both Part A and Part B for the entire 12-month period unless death was the cause of discontinuation, and were not a member of an Health Maintenance Organization (including Medicare Advantage) in any given month. This gave us 9,522 PCPs with a total of 1,107,153 attributed patients. We also dropped patients and PCPs with missing values in beneficiary and physician characteristics, respectively, and those with missing ZCTA crosswalk information. This reduced the PCP sample by 2.5% to 9,243 PCPs with 1,095,783 attributed patients. We also dropped those PCPs that had an attributed patient panel too small—those with less than 20 patients—to determine the average spending level of their attributed patients. Lastly, 1 PCP with a missing weight was dropped from the sample. This led to the final sample size of 6,873 PCPs with 1,078,840 attributed patients.
Measures of LVC Services
From the list of low value services identified by the Choosing Wisely Campaign, we first selected those services that were relevant to primary care and to the elderly population. Using the measures developed by Colla et al.6 and Schwartz et al.12, we then identified 8 primary care-associated LVC services that could be measured using the Part B Claims data (carrier line file). Specifically, for each LVC service type, we first identified qualifying patients who were eligible for the service based on diagnoses, demographics, and other criteria from all patients that had received the service. We then excluded those patients whose receipt of service could be “justified” based on their medical history, as reported in the carrier claims, before the service date. Therefore, for each LVC service type, the services provided to type-specific qualifying patients that did not satisfy the exclusion criteria were deemed LVC.
Covariates
To take into account the differences in patient panel composition across PCPs that may influence LVC use, we adjusted for demographic characteristics in terms of age, race/ethnicity, and sex and health status distributions of the PCP's patient panel in the model. Patient age was categorized into 5 bins with the first 4 bins in 5-year increments from age 65, while the last 1 included all patients who were 85 and older. The share of patients in the patient panel in each category was calculated. Similarly, race and ethnicity were categorized into white, black, and all others. ElixHauser Comorbidity Index conditions, which are a set of dichotomous health condition categories created using the International Classification of Diseases diagnoses codes that predict inpatient metrics in terms of the length of hospital stay, mortality, and costs were used to adjust for the health distribution of the patient panel. The shares were calculated for 4 categories of the number of present ElixHauser Comorbidity conditions. We also included ZCTA-level socioeconomic and demographic characteristics of practice location to adjust for differences in communities they serve. This included the percentage of those aged 25 and older with less than high school education, percentages of Hispanic and Black population, and percentage of those with income less than 200% of the federal poverty line.
Sensitivity Analyses
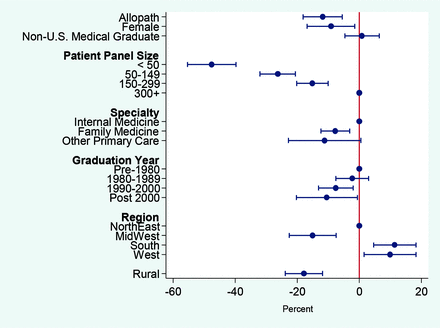
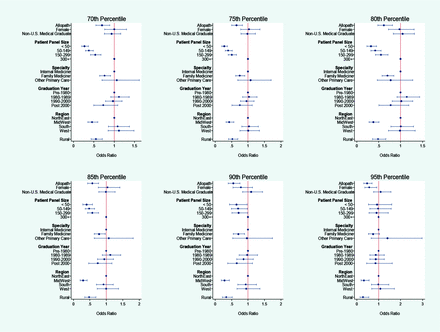
Several sensitivity analyses were performed to test the robustness of our findings. First, to address the concern that Medicare payments may not represent the actual costs of LVC services due to patient copayments, deductibles, and third-party payments, we tested whether the results for physician characteristics held when per-patient LVC allowed charges were used as an alternative outcome. As Appendix Figure 1 shows, there were no qualitative differences in the findings when per-patient allowed charges was used. Second, the long right-side tail of the PCP sample distribution of per-patient LVC Medicare spending in Appendix Figure 1 indicated that a small number of PCPs with “extremely high” LVC spending could be driving the results. However, a sensitivity analysis using the log per-patient LVC Medicare spending as an outcome showed similar patterns as our main regression results, implying that the results were not driven by outliers (Appendix Figure 2). Third, because our study only included LVC services reported in claims by noninstitutional providers, we omitted either some or all the costs of LVC received in institutional settings, such as hospital outpatient departments and federally qualified health centers. PCPs that work primarily in safety-net institutions, such as federally qualified health centers and rural health clinics, would be mostly excluded from our sample. However, when we stratified LVC services by likely place of service, ones that were likely to take place in an office versus in a facility, the results showed no systematic difference (Appendix Figure 3). Lastly, to check whether the findings for the high LVC group using the logit model were robust to the per-patient LVC spending thresholds, we varied the threshold from the top 30th to 10th percentiles in increments of 5 in defining the high LVC spending group. The estimates for allopath, rural, practice region, and physician specialty were robust to changes in thresholds of per-patient spending for the high LVC group, whereas the results for patient size did not hold when the threshold was moved to the top decile (Appendix Figure 4).
Sensitivity Analysis for OLS Regression Results using per-patient Low Value Care Medicare Allowed Charges ($) as Outcome.
Note: The dependent variable in this OLS regression was PCP's per-patient LVC allowed charges in 2011. Per-patient LVC allowed charges were computed by adding up all the allowed charges associated with LVC services received by their attributed Medicare patients and then dividing by the number of attributed Medicare patients. As in our main OLS model, the model was estimated using sample weights to reflect the oversampling of physicians in smaller states. The model was adjusted for patient composition with respect to age, sex, race/ethnicity and Elixhauser Comorbidity Index, and for PCP's practice location characteristics including percent black, percent Hispanic, percentage of those aged 25 and older with less than high school education, and percentage of those with income less than 200% federal poverty line (FPL).
Sensitivity Analysis for OLS Regression Results using Logarithm of Per-Patient Low Value Care Medicare Spending as Outcome.
Note: The dependent variable in this OLS regression was PCP's log per-patient LVC Medicare spending in 2011. As in our main OLS model, the model was estimated using sample weights to reflect the oversampling of physicians in smaller states. The model was adjusted for patient composition with respect to age, sex, race/ethnicity and Elixhauser Comorbidity Index, and for PCP's practice location characteristics including percent black, percent Hispanic, percentage of those aged 25 and older with less than high school education, and percentage of those with income less than 200% FPL.
Sensitivity Analysis for OLS Regression Results—Association between Physician Characteristics and Per-Patient LVC Spending ($) by Place of Service.
Note: The model was estimated using sample weights to take into account the oversampling of physicians in smaller states. Regions and rurality were determined based on the PCP's practice location. The model was adjusted for patient composition with respect to age, sex, and race/ethnicity. Patient age was categorized into 5 bins with the first 4 bins in 5-year increments from age 65, while the last 1 includes all patients who were 85 and older. The 3 race/ethnicity categories included were white, black, and all other race/ethnicity. ElixHauser Comorbidity Index conditions were used to adjust for health distribution of the patient panel. The model was also adjusted for PCP's practice location characteristics including percent black, percent Hispanic, percentage of those aged 25 and older with less than high school education, and percentage of those with income less than 200% FPL. See Appendix Table 2 Panel A for coefficient estimates of all the variables included in the model.
Robustness Check for Logistic Regression Results with Respect to Changes in Thresholds for Identifying High LVC Spending Group.
Note: The reported estimates are odds ratios from logit models with varying cutoff points for the binary outcome of whether PCP had a high LVC spending in 2011. For example, for the 70th percentile, the binary dependent variable was equal to 1 if the PCP's per-patient LVC spending in dollar amounts was in the top 70th percentile. As in our main logit model, the models were estimated using sample weights to take into account the oversampling of physicians in smaller states. The models were adjusted for patient composition with respect to age, sex, race/ethnicity and Elixhauser Comorbidity Index, and for PCP's practice location characteristics including percent black, percent Hispanic, percentage of those aged 25 and older with less than high school education, and percentage of those with income less than 200% FPL.
Low Value Care Prevalence Rate and Associated Medicare Spending ($)*
Association between Primary Care Physician Characteristics and Low Value Care Spending of Attributed Medicare Patients
Association between Practice Location Rurality and Low Value Care Spending of Attributed Medicare Patients Across Specialty
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
To see this article online, please go to: http://jabfm.org/content/32/2/218.full.
- Received for publication April 8, 2018.
- Revision received November 5, 2018.
- Accepted for publication November 30, 2018.