Abstract
Purpose: Left ventricular hypertrophy (LVH) is common in primary care and is associated with increased morbidity and mortality. Treatment of underlying hypertension can reverse LVH and eliminate the associated risks. Electrocardiography is widely available and commonly used to screen hypertensive patients for LVH, but it is limited by low sensitivity. Limited echocardiographic measurement of the left ventricle is a method for screening with improved sensitivity; however, it is not currently widely used in the primary care setting. This study attempts to test the accuracy of primary care physicians' (PCPs) measurements of the left ventricle using a pocket-sized ultrasound (pUS) device after a brief training session.
Methods: This study was performed in an outpatient cardiology clinic by 3 family medicine residents and 1 family medicine faculty member after a 4-hour training session. Measurements of the left ventricle were made by PCPs using a pUS device; these measurements were compared with cardiologists' measurements from images obtained by echocardiography technicians. Left ventricular mass index (LVMI) was calculated based on these measurements and then compared between groups.
Results: There was no statistically significant difference between the mean LVMI calculations in the 2 groups. The agreement in measurements between the groups, however, showed high variability. This was manifested by the low sensitivity (70%) and specificity (76%) of PCPs in the detection of LVH.
Conclusions: This study showed that limited echocardiography for the detection of LVH performed by PCPs at the point of care was feasible. Future studies are needed to determine the ideal training and experience necessary to yield competency.
Left ventricular hypertrophy (LVH) is a comorbidity present in approximately 14% of patients with untreated systemic hypertension.1 It is associated with increased rates of sudden cardiac death and cardiovascular morbidity.2,3 Regression of LVH occurs with treatment of hypertension and is associated with a decrease (36%) in the relative risk of cardiovascular morbidity.4 Guidelines from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure called for screening of all patients with newly diagnosed hypertension using electrocardiography (EKG) at the time of diagnosis.5 These recommendations were not updated in Eighth Report of the Joint National Committee.
EKG has suboptimal sensitivity (54%) and specificity (86%) when testing for LVH.6 Echocardiography has high sensitivity (88%) and specificity (84%), but it is limited as a routine screening tool because of its high cost, its limited availability, and the need for specialized training.6 Limited echocardiography is a tool that may be used to overcome these barriers.7 It includes only those components of an echocardiographic examination needed to assess for LVH.8
Despite the potential benefits of limited echocardiography, cost and availability are still the main factors limiting widespread use. EKG is still the predominant tool used to screen for LVH. However, there is potential for increased use of limited echocardiography with the availability of an inexpensive and highly portable ultrasound such as the pocket ultrasound (pUS). These devices have an accuracy similar to that of traditional machines when used by experienced cardiologists.9
The pUS has shown potential for effective use outside of the specialty setting. Primary care physicians (PCPs) accurately used pUS devices, after minimal training, to assess left ventricular systolic function, an examination similar in complexity to evaluation of LVH.10 However, whether PCPs with minimal training can use pUS to calculate the left ventricular mass index (LVMI), which is needed to screen for LVH, is not currently known. Our study was developed to evaluate whether PCPs can accurately assess LVMI at the bedside equivalently to evaluations by cardiologists using routine echocardiography.
Methods
This study was conducted at the Tripler Army Medical Center, Honolulu, Hawaii. The study protocol was approved by the Human Use Committee at Tripler Army Medical Center. Investigators adhered to the policies for protection of human subjects as prescribed in the Code of Federal Regulations title 45, part 46.
Training
Four PCPs (1 family medicine staff physician and 3 family medicine residents) were trained in limited echocardiography. All had no prior experience or training in performing echocardiograms (see Table 1).
All PCPs received a total of 4 hours of supervised training. Initial training consisted of 73 minutes of online modules from the Society of Ultrasound in Medical Education (http:www.susme.org/learning-modules/learning-modules); these included Introduction to Ultrasound, Introduction to Ultrasound Transducers, Image Orientation and Resolution, Introduction to Ultrasound Artifacts, Bio-Effects of Ultrasound, Introduction to Cardiac Ultrasound, and LVH Screening in Patients with Systemic Hypertension. In addition, online training included a single module from the manufacturer of the pUS: Introduction to Vscan Portable Ultrasound Device (https://vscan.gehealthcare.com/vscan-clinical-education#/gallery/introduction-to-vscan-portable-ultrasound-device).
The remainder of the 4-hour training session was dedicated to hands-on practice of these methods with a pUS device (Vscan; GE Healthcare, Milwaukee, WI). All hands-on training was led by the principal investigator (PB), a family physician credentialed in limited echocardiography. The PCPs also had access to the pUS to practice on their own after this initial training, and they were allowed unlimited access to the online training modules. The PCPs were allowed to participate in the study after completing this training. No further assessment of competency was performed.
Power Analysis
A power analysis was done to determine the appropriate sample size. It was determined that with a sample size of 100 paired measurements from 100 patients, letting α = 0.05, the study would have 80% power to detect a difference of 10 g/m2 between the pUS and echocardiogram measures of LVMI, assuming a standard deviation of 343 and a correlation of 0.5 between the 2 values.
Data Collection
Hospitalized patients and outpatients scheduled for an echocardiogram in the cardiology clinic were screened, along with their outpatient medical records, for meeting inclusion criteria. Inclusion criteria were age ≥18 years and history of blood pressure measurements ≥140 systolic and/or ≥90 diastolic on 2 separate readings at least 24 hours apart. Eligible patients were approached while waiting in the cardiology clinic or hospital wards and asked to participate in the study. Written informed consent was obtained. Patients were excluded from the final analysis if no interpretable images could be obtained with the pUS.
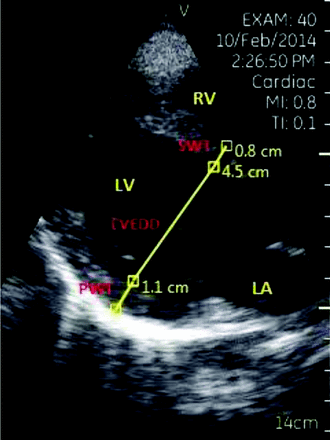
Enrolled patients received a full echocardiogram performed by an echocardiography technician on a high-end ultrasound machine that included left ventricular measurements of septal wall thickness (SWT), posterior wall thickness (PWT), and left ventricular end diastolic diameter (LVEDD) obtained by B-mode images from the parasternal long-axis window (see Figure 1). After the echocardiogram was completed, the PCP performed the pUS examination with the patient within 14 days of the initial examination. PCPs were blinded to the patients' echocardiographic results and medical records. Measurements and images from both examinations were stored digitally in the cardiology clinic and on the pUS machines. A staff cardiologist interpreted the stored echocardiogram images and verified measurements obtained by the echocardiography technician, which then were stored in a diagnostic report in the patient's medical record.
Parasternal long-axis view of the heart with measurements. LA, left atrium; LV, left ventricle; LVEDD, left ventricular end diastolic diameter; PWT, posterior wall thickness; RV, right ventricle; SWT, septal wall thickness.
All data were compiled into a database by the principal investigator and were used to calculate the LVMI and to determine the presence or absence of LVH based on predetermined cutoffs of LVMI for both the PCP and cardiologist measurements. The primary end point in this study was the mean difference in LVMI between the 2 groups. LVMI was calculated from measurements recorded by the cardiologists and the PCPs. The left ventricular mass was calculated based on American Society of Echocardiography guidelines with Deveroux's formula11:
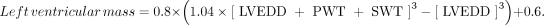
The left ventricular mass then was divided by the patient's calculated body surface area to determine the LVMI.
Secondary end points included comparisons of average LVEDD, SWT, and PWT measurements between the cardiologist and PCP groups. Average LVEDD, SWT, and PWT and calculated LVMI documented by each individual cardiologist and PCP also were analyzed to determine whether there were any differences based on the examiner. Each PCP's total number of pUS examinations performed was compared with the overall difference of means for the PCP and cardiologist groups to determine whether the number of exams completed had an effect on the accuracy of the measurements.
Body mass index (BMI), age, and sex were obtained from the most recent medical record entry for each patient. These were used to perform subgroup analysis of average LVEDD, SWT, and PWT and calculated LVMI between the cardiologist and PCP groups to determine whether these factors introduced bias into the calculations.
Sensitivity and specificity of the PCP examination for diagnosis of LVH were calculated using the cardiologist examination as the gold standard. LVH was defined as LVMI >95 g/m2 for women and >115 g/m2 for men.11
Data Analysis and Statistics
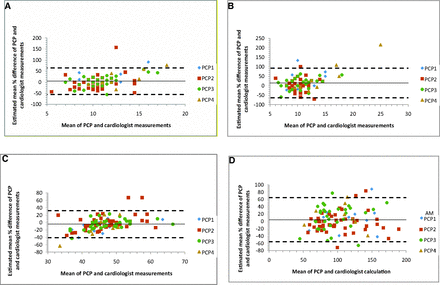
Bland-Altman charts were produced to assess the accuracy and precision of the PCPs' measurements compared with the cardiologists' measurements of LVMI.12 A 2-sided paired t test was used to estimate bias. Adjusted linear models were created to see whether bias was associated with patient age, sex, or BMI. Results were calculated overall and stratified by PCP and cardiologist. A significance level of 0.05 was used for all analyses. All statistical analyses were done using SAS software version 9.2 (SAS Institute, Cary, NC).
Results
Of the 106 patients who agreed to participate, 5 were excluded from the final analysis because of an inability to obtain interpretable images on the pUS. A total of 101 patients were included in the final analysis. The characteristics of these patients are shown in Table 2.
The differences in the means of measurements of SWT, PWT, and LVEDD, as well as for the calculation of LVMI, were compared between the cardiologist and PCP groups. There were no statistically significant differences found for LVMI (mean difference, 0.5 g/m2; P = .885) or for SWT (mean difference, 0.4; P = .232). There were statistically significant differences for PWT (mean difference, 1.2; P = .007) and LVEDD (mean difference, −2.1; P = .013). These findings are listed in Table 3. Results were then compiled for individual PCPs and cardiologists, which showed among these examiners no statistically significant outliers that could be skewing the data. Adjusted linear models showed that there was no bias associated with patient age, sex, or BMI.
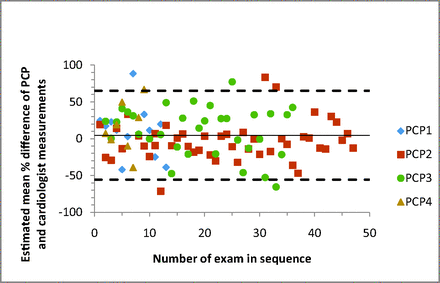
Bland-Altman plots were created to illustrate agreement in measurements for individual data points between the cardiologist and PCP groups. Variability was high, and confidence intervals for the distribution of LVMI ranged from −56% to 65% of the mean of the paired measurements (Figure 2). Plots also were created to compare the agreement of measurements done by each individual PCP. There was no statistically significant difference found. Variability was lower for PCP2, the 1 family medicine faculty member in the group, but this was not statistically significant (P = .14). Differences were also plotted for individual PCPs versus sequence number to determine whether agreement changed with an increasing number of examinations performed (Figure 3). Here we noted that agreement for PCP2 (the only PCP to complete >37 exams) was significantly better for the last 10 readings compared with the first 37 (P = .04). These points were arbitrarily chosen, however, and statistical significance would be lost if the cutoff was moved slightly—such as looking at the last 12 exams.
Bland-Altman plots of differences in mean measurements of septal wall thickness (A), posterior wall thickness (B), left ventricular end diastolic diameter (C), and left ventricular mass index (D) between primary care physician (PCP) and cardiologist groups. In each graph, the dotted lines represent the 95% confidence intervals of the percentage difference between PCPs and cardiologists. The solid line represents the estimated mean difference. The legend to the right identifies the different PCP examiners.
Bland-Altman plot of the difference in mean calculations verus sequence number. The dotted lines represent the 95% confidence intervals of the percentage difference between the primary care physicians (PCPs) and cardiologists. The solid line represents estimated mean difference. The legend to the right identifies the different PCP examiners.
Sensitivity and specificity, along with positive and negative predictive values and likelihood ratios, were calculated for PCP exams using the cardiologist examination as the gold standard. These values are depicted in Table 4.
Discussion
Our hypothesis was that there would be no difference in calculations of LVMI between PCP and cardiologist groups, and our results indeed showed no statistically significant difference. The lack of statistical significance could be related to the relatively small sample size. However, our study was powered to detect a difference of LVMI of 10 g/m2. We found a mean difference for LVMI of only 0.5 g/m2, which is very low, so failure to find a significant difference was not likely the result of a lack of power.
Although there was no statistically significant difference in mean LVMI, the variability was large, as evidenced by the confidence on the Bland-Altman plots (Figure 2A–D). The effect of this variability can be seen when analyzing the sensitivity (73%) and specificity (75%) for detecting LVH. These sensitivity and specificity values would not normally be considered characteristics of a good clinical test, in which these values would normally be above 80%.
It is possible that this variability could be from the PCPs obtaining images that were inaccurate or inaccurately performing measurements on these images. It would have been ideal to have the cardiologists who served as the reference standards also review the PCPs' images to determine whether the error was in image acquisition or the measurements. This was not possible in this study, but the images obtained by the PCPs were reviewed by the study's principle investigator, who also provided the training to the PCPs, and most of the error was considered to be the result of image acquisition.
Variability does seem to decrease with the number of exams performed by the PCPs, and this is statistically significant above 35 exams. This is also evidenced by an improving value for the sensitivity of detecting LVH, which increased to 88%, although there was a slight decrease in specificity (67%). It is important to note that only 1 PCP performed more than 35 exams (PCP2), and he also had an overall trend toward less variability in all exams performed, though this was not statistically significant. In addition, 35 exams is an arbitrarily selected cutoff. It was selected after searching for a number above which there would be a statistically smaller amount of variability. It cannot be guaranteed that this would continue if further examinations were performed.
The PCPs were not given any feedback on the echocardiograms they were performing after the initial training. After the completion of the study, the PCPs voiced that they did not initially feel comfortable with their measurements and that it would have been helpful to have further feedback when they first started performing these exams. It is our belief that if the initial amount of training provided to the PCPs was increased, and a limited number of supervised echocardiograms occurred following the training, there would have been a large improvement in the variability.
Another possible limitation to the study is that the “gold standard” used here was another test: an echocardiogram performed by a technician and interpreted by a cardiologist. It is known that differences in patient position, transducer placement, and interobserver variability can effect agreement between observers.13 Although echocardiography has been validated as an accurate test of left ventricular mass when compared with autopsy measurements, these studies followed strict protocols in regulating these variables.7 While the PCPs in this study did have protocols regarding patient position and probe placement, there was no way to be sure that echocardiography technicians were following similar protocols. It is possible that these differences may have caused additional variability in our results, with a loss of agreement between observers.
As indicated in Figure 2A—D, there seems to be an upward linear trend. For smaller values, the PCPs' measurements tended to be less than the cardiologists', and for higher values, they tended to be higher. This may suggest that the echocardiography technicians were aware of a normal value for each measurement and may have been hesitant to record a measurement that was too different from the normal. This should have been corrected by the cardiologist when the images were reviewed; however, one cardiologist who participated in this study admitted that sometimes when the technicians' measurements were close, they were not always changed if it did not make a difference in patient care.
These potential limitations may have been better accounted for if the patients were evaluated by the PCP and the echocardiography technician in a controlled environment where standard protocols were followed, instead of the high-demand environment of a busy clinical practice. In addition, even among highly trained sonographers, inter-reader variability as high as 23.8% has been documented for measurements of left ventricular mass.14 In future studies it may be beneficial to have the same patients evaluated by multiple PCPs and cardiologists to determine inter-reader variability and to determine whether it is a factor in any variability.
Given these limitations, we still believe this is a valuable study. It demonstrates that point-of-care ultrasound measurement of left ventricular mass by a PCP is feasible, although further studies that include protocols with more training and greater initial supervision are needed.
Acknowledgments
The authors thank Thomas Wisenbaugh, MD, for his help in developing the design of this study and the entire Tripler Army Medical Center Cardiology Division for all the help they provided.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
Disclaimer: The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the US Government.
- Received for publication November 11, 2014.
- Revision received May 21, 2015.
- Accepted for publication May 29, 2015.