Abstract
Background: Federally qualified health centers (FQHCs) can address high rates of unintended pregnancy among adolescents in the United States by increasing access to intrauterine devices (IUDs) in underserved settings. Despite national guidelines endorsing adolescent use of IUDs, some physicians remain concerned about IUD tolerance and safety in adolescents. Therefore we compared adolescents and adults in a family physician staffed FQHC network with regard to (1) IUD postinsertion experience, (2) device discontinuation, and (3) sexually transmitted infection (STI) rates.
Methods: We conducted a retrospective cohort study among women <36 years old who had an IUD inserted in 2011 at a New York City FQHC staffed by family physicians.
Results: We included 684 women (27% adolescents, 73% adults). During the 6-month postinsertion period, 59% of adolescents and 43% of adults initiated IUD-related clinical contact after insertion, most commonly for bleeding changes and pelvic or abdominal pain. There were no significant differences between groups in IUD expulsion or removal or STI rates.
Conclusions: Urban FQHC providers may anticipate that, compared with their adult IUD users, adolescents will initiate more clinical follow-up visits after insertion. Both groups will, however, have similar clinical concerns about, reasons for, and rate of device discontinuation and low STI rates.
Unintended adolescent pregnancy in the United States is associated with substantial health, educational, social, and economic costs and has been identified by the Centers for Disease Control and Prevention as 1 of the top 6 “winnable” public health battles.1 Approximately 4 of 5 pregnancies among US women ≤19 years old is unintended; overall unintended pregnancy rates are highest among poor and low-income women.2 Federally qualified health centers (FQHCs), designed to provide primary care (including reproductive health services) for populations with disproportionate barriers to health care—including women, the uninsured, and ethnic and racial minorities—can play a significant role in addressing this issue through the use of primary care physicians and an infrastructure of support staff and ancillary services.3⇓–5
Intrauterine devices (IUDs), which are among the most effective reversible contraceptives,6 can be inserted during routine office visits at FQHCs.5 However, while professional clinical guidelines endorse the safety and effectiveness of IUDs in adolescents and recommend increased access to this contraceptive,7 of which there are options both containing hormones (levonorgestrel) and not containing hormones (copper), <5% of 15- to 19-year-old contraceptive users currently use IUDs.8 Established challenges to providing IUDs include limited access to providers trained in insertion (including those at FQHCs)5 and practitioner concerns that, compared with adults, adolescent IUD users will (1) not tolerate expected device-related side effects, (2) experience more expulsion, especially because of nulliparity, and (3) have increased risk of sexually transmitted infections (STIs), including pelvic inflammatory disease.9⇓⇓⇓⇓⇓⇓⇓–17
While there exist studies primarily addressing these concerns among insured adolescents and adolescents using specialty clinics,5,18⇓⇓⇓–22 there are no studies examining adolescent IUD users' outcomes and postinsertion clinic use in FQHCs staffed by family physicians. These data are of particular importance because the Affordable Care Act has allocated $11 billion in funding for expansion and operations of community health centers for the underserved over the next 5 years, and family doctors serve as the largest proportion of the physician workforce in FQHC settings.3,23 Thus, the aim of our study was to further prepare FQHCs for comprehensive contraceptive management by addressing common provider concerns regarding IUDs in adolescents and by examining the clinical needs of adolescent patients after insertion. Specifically, our study compares the 6-month experience after IUD insertion between adolescents and adults in an FHQC network staffed by family physicians with regard to (1) frequency and content of patient-initiated follow-up with physicians regarding device-related issues, (2) device discontinuation, and (3) STI rates.
Methods
Setting
This study was conducted at the Institute for Family Health (IFH), an FQHC network in New York State that provides full-spectrum primary care in the Bronx, Manhattan, and the Mid-Hudson Valley. In 2011, family physicians inserted IUDs at 11 of 26 IFH sites, including one location that houses the Beth Israel Residency in Urban Family Practice program. Since February 2009, the residency site has provided free grant-funded IUDs for uninsured patients and adolescents requiring confidential IUD insertion. This site also is affiliated with a high school–based health center.
Sample
All female patients ≤35 years old who had an IUD inserted by a family physician at an IFH site during 2011 were included in this study. Adolescents were defined as patients <21 years old on the day of insertion. We selected 35 years as the upper age for inclusion, given that women of older ages may have decreased fecundity24,25 and thus may be more likely to have noncontraceptive reasons for IUD use and discontinuation. However, use of the IUD for noncontraceptive indications was not a criterion for exclusion, despite possibly altering the patient's motivation for continuing use of this method. For participants who had >1 IUD inserted in 2011, only information from their first 2011 insertion visit was included.
Design
We conducted a retrospective chart review of all IUDs inserted in 2011 (insertion visit). Each chart was reviewed from the date of insertion for up to 6 months after insertion or until the device was discontinued, which ever occurred first, to record all patient-initiated contacts with providers during the study period. Charts of patients who did not have any contact with a provider during the 6-month study period were further reviewed for an additional 6 months (for a total follow-up of 1 year from the time of insertion) to determine whether the IUD remained in place or whether there was reference to discontinuation during the initial 6-month study period. We selected a 6-month follow-up because previous studies showed that most IUD users who either remove or expel their device will do so in the first 6 months after insertion.22,26
Data Collection
This study was approved by the IFH Institutional Review Board. Potential participants were identified via International Classification of Diseases, Ninth Revision, and Current Procedural Terminology codes in billing data. Data were extracted from the electronic medical record and entered into a database. Via the electronic medical record interface, primary care office visits, telephone encounters, and electronic communications between patients and providers were reviewed using the search terms IUD, contracept, mirena, paragard, copper, pid, and pelvic inf. Information was included in the study if it was determined that the patient initiated contact with the provider for an IUD-related concern. Of note, there is a protocol in place for patients from the affiliated high school who have IUD insertions, whereby a clinic social worker initiates follow-up contact at 1 day, 1 week, 6 weeks, 3 months, and 6 months after IUD insertion to determine whether the patient is satisfied. These encounters were not included in the study because they were not initiated by the patient.
Measures
Insertion Visit
Baseline visit information included the following variables: age, race (self-report), ethnicity (self-report), gravidity, parity, IUD type, IUD payment method (insurance or grant funded), reason for insertion (answers were not mutually exclusive), and timing of gonorrhea and chlamydia testing (none, within 2 weeks before insertion, or on the day of insertion).
Follow-up Visits
Information about patient-initiated follow-up visits included contact type (office visit/telephone/electronic communication); reason for contact; whether a removal occurred and, if so, the reason for removal; whether expulsion was identified; and whether a gonorrhea and chlamydia test was ordered and test results. IUD providers at IFH typically do not mandate postinsertion follow-up after a set period of time; instead, they recommend that patients contact physicians as needed for IUD-related concerns.
Discontinuation
Discontinuation was defined as either IUD expulsion or removal. If a removal or expulsion occurred outside of an IFH encounter, the date of discontinuation was entered as the date of the patient-initiated contact whereby the provider first became aware of the event.
Data Analysis
Descriptive statistics were tested using χ2 tests, t tests, and Wilcoxon signed-rank tests, as appropriate, with significance defined as P < .05. Separate survival models were developed to assess time until removal and time until expulsion, and Kaplan-Meier tests were used to allow for censored data comparing adolescents and adults.
For patients who had follow-up visits that indicated IUD removal elsewhere and did not have the date of removal or expulsion recorded in the chart, the mean time until removal for known cases was imputed such that the date of the office visit was recorded as the date of IUD discontinuation. Patients who had no follow-up during the time period assessed were examined with 2 different models. In the first model, all patients with no further follow-up were categorized as “lost to follow-up,” so they did not contribute to the survival model. The second model was assessed under the assumption that all patients with no further contact were satisfied with their device and that their IUDs remained in place at 6 months.
Results
Demographics
A total of 684 patients were included in this study: 182 adolescents (27%) and 502 adults (73%) (Table 1). The proportion of adolescents <18 and 18 to 20 years old were similar (51% and 49%, respectively); the 2 youngest patients were 13 years old. The age distribution of adults showed that there were similar proportions of patients in the categories of 21 to 25 and 26 to 30 years old (38% and 40%, respectively), whereas there were relatively fewer adults aged 31 to 35 years (22%). Adolescents and adults had significantly different race and ethnicity distributions; notably, a greater proportion of adolescents than adults reported “mixed race” (41% vs 29%, respectively) and Hispanic ethnicity (45% vs 37%, respectively). Adolescents were more likely to be nulligravid and nulliparous than the adult women: 35% of adolescents reported ever being pregnant, and 11% reported a pregnancy resulting in childbirth, whereas 63% of adults reported ever being pregnant and 46% reported a pregnancy resulting in childbirth. Thirty percent of patients—and a significantly greater proportion of adolescents compared with adults (45% and 24%, respectively)—received grant-funded IUDs. Of insertions, 78% in adolescents and 67% in adults occurred at the residency training site; this difference was statistically significant (P < .001).
There was no significant between-group difference in selection of IUD type; 72% of adolescents and 71% of adults chose the hormone-containing device, and 28% of adolescents and 29% of adults chose the copper-containing IUD. The most common reason for IUD insertion, which was similar between both groups, was for routine contraception (72% of adolescents and 67% of adults). Adolescents were more likely than adults to have their IUDs inserted after an abortion (21% of adolescents and 13% of adults; P = .01), whereas adults were more likely to have their IUDs inserted postpartum (3% of adolescents and 12% of adults; P < .01).
Frequency and Content of IUD-Related, Patient-Initiated Follow-up
During the 6-month postinsertion period, adolescents were significantly more likely to initiate IUD-related contact with a provider compared with adults (59% of all adolescents and 43% of all adults; P < .001). The only baseline characteristic that differed significantly between patients who did and did not have IUD-related, patient-initiated contacts in the 6 months after insertion was IUD payment type; a greater proportion of adults with insurance-paid IUDs initiated contact compared with adults with grant-funded IUDs (P < .001). Of note, among the 7 women who received IUDs for menorrhagia, 6 had no further follow-up after insertion.
Among patients who initiated at least 1 IUD-related follow-up visit in the 6 months after insertion, adolescents made more visits (median of 2 for adolescents vs 1 for adults) and were more likely to have repeated contacts; 37% of adolescents and 15% of adults initiated ≥3 contacts with providers during the follow-up period. The most common reasons for contact were similar between the 2 groups and included bleeding changes, pelvic or abdominal pain, string check, and request for removal (Table 2).
IUD Discontinuation
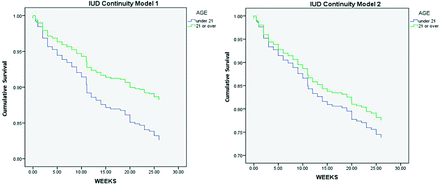
Device continuation was assessed with 2 survival analysis models, as described in the Methods (Figure 1). Both models showed no significant differences in IUD continuation during the 6-month postinsertion period when comparing adolescents and adults (model 1: hazard ratio, 1.54 [95% confidence interval, 0.99–2.40]; model 2: hazard ratio, 1.21 [95% confidence interval, 0.78–1.89]).
Comparison of 6 month device continuation rates between adolescents and adults who had an IUD inserted at the IFH FQHC network in 2011.
In addition, continuation rates based on site of insertion were compared within age groups and showed that for both adolescents and adults, nonresidency sites had a relatively greater percentage of removals and expulsions compared with a residency site of insertion (29% and 25%, respectively, among adolescents and 24% and 23%, respectively, among adults).
Expulsions
Of the patients who initiated IUD-related follow-up, there was no significant difference in the proportion of expulsion when comparing adolescents and adults (9% and 6%, respectively), even in subgroup analysis by device type, gravidity, and parity (P = .20, .70, and 0.50, respectively), with the assumption that those who had no further contact with the clinic after IUD insertion retained their device at 6 months (Table 3).
Removals
No significant difference in the proportion of IUD removals at 6 months was found when comparing adolescents and adults (17% in both groups) who initiated IUD-related follow-up, which remained consistent in subgroup analysis by device type, gravidity, and parity (P = .73, .12, and .11, respectively), with the assumption of device retention as noted above (Table 3). In both age groups, most IUDs were removed because of pain or bleeding. The median number of contacts before IUD removal showed adolescents may have tended to have at least 1 additional visit before removal compared with adults (3 vs 2, respectively). Of note, 1 IUD removal occurred in a 23-year-old nulligravid woman with a copper IUD secondary to pregnancy. This was the only event of pregnancy among IUD users found in our study.
STI Rates
Overall, a significantly greater number of adolescents had STI testing before insertion compared with adult users (P < .01), whereas rates of testing after insertion were similar between age groups (P = .75) (Table 4). Similar low rates of infection were noted among both adolescents and adults (P = .91and P = .70, respectively); chlamydia was found to be relatively more common than gonorrhea both on the day of insertion (in both groups approximately 3% had chlamydia and <1% had gonorrhea) and during the postinsertion period (4% chlamydia in adolescents and 5% chlamydia in adults, with no cases of gonorrhea in either group). Of note, one adult was treated empirically for pelvic inflammatory disease based on symptoms and examination findings (gonorrhea and chlamydia testing were negative). Her IUD was not removed during this time.
Discussion
This study addressing gaps in contraceptive research about adolescents' experience with IUD discontinuation and side effects is the first to do so exclusively among family physician IUD providers in the rapidly growing FQHC setting.27 Our results show that there are no significant differences in IUD expulsion or removal rates between adolescents and adults and suggest that IUD users from both groups have similar experiences 6 months after insertion. Based on our findings, urban FQHC providers may anticipate that, compared with their adult patients with IUDs, adolescents with IUDs will initiate more clinical follow-up visits after insertion. However, both groups will have similar clinical concerns, reasons for device discontinuation, and low rates of STIs.
With regard to device continuation, our first survival model, which assumed all IUD users were lost to follow-up, borders on a significant difference in IUD retention between age groups; however, the true value likely lies between our findings for models 1 and 2 (which assumed device retention in all patients lost to follow-up), ultimately illustrating no significant change in continuity based on age, which is consistent with studies of expulsions and removals in varied settings and populations.19,25,28
Our patients' experiences with follow-up visits for pain and bleeding changes mirror findings in non-FQHC settings, including family medicine clinics, as described by Dickerson et al,29 who surveyed adult women about their most common IUD side effects and reasons for early removal of the device. Studies also suggest that patient education regarding these expected concerns may result in decreased device continuation rates. For example, the study of urban women with IUDs by Garber et al26 found that those who met with health educators were less likely to experience early discontinuation compared with women who did not meet with a health educator. Given that FQHC infrastructure can allow for a multidisciplinary team approach to care, the incorporation of health educators may be a feasible and efficacious component of IUD management, potentially decreasing patient concern after insertion, device longevity, and physician utilization.
The STI rates among adolescent IUD users in our study are comparable to national STI rates among girls 15 to 19 years old.30 The low STI rates in our study are consistent with a prior study of adolescent IUD users across a variety of settings.20
Alton et al20 examined IUD experiences among young women up to age 21 at 3 different sites—a private practice, a Title X clinic, and a community-based, grant-funded clinic with a high-risk adolescent population—and found that IUDs did not increase the risk of infection. Thus, our findings should further reassure primary care providers about the safety of IUDs for adolescents with regard to infection, and they can be used to support the practice of STI testing on the day of insertion (instead of requiring results before insertion), thereby decreasing additional barriers to the use of this effective contraceptive method.31,32
Study Limitations
Methodologically, given the high school health clinic protocol of contact initiated by a social worker after IUD insertion, it is unclear whether these interactions ultimately decreased adolescent patient-initiated office visits through reassurance of common concerns or increased office contacts for issues that patients may not have otherwise sought out a provider based on the social worker's recommendation.
In addition, limited power did not enable us to control for the baseline variation between adolescents and adults, particularly with regard to gravidity and parity, or to conduct postinsertion multivariate analysis to determine characteristics associated with device discontinuation. In addition, because of loss to follow-up, our findings with regard to patient-initiated IUD follow-up and STI diagnoses are likely underestimates because patients may have initiated care elsewhere after insertion.
Conclusions
Three key areas of further research include (1) investigating how provider practices manage adolescent and adult IUD removal requests, given the unclear causation for our finding that adolescents tended to initiate an additional visit with a provider before device removal compared with adults; (2) investigating patient-related outcomes with regard to additional methods of long-acting reversible contraception, such as implantable devices, in FQHC settings; and (3) examining the short- and long-term impact of family medicine residency procedural training in FQHC settings on access to effective contraception for underserved populations.33
If the Centers for Disease Control and Prevention's “winnable goal” of decreasing unintended adolescent pregnancy rates is to be met, increasing IUD promotion by primary care physicians and expanding FQHC infrastructure to support IUD insertions (particularly given the expected increase in FQHCs staffed by family physicians under the Affordable Care Act) are needed. Results from our study can encourage family physicians to offer IUDs to adolescents in urban FQHCs and to anticipate the follow-up needs of their patients after insertion. These efforts will continue to emphasize the leading role of family physicians in addressing unintended adolescent pregnancy.
Notes
This article was externally peer reviewed.
Funding: This study was funded in part by grant support from the American Academy of Family Physicians. SER's salary is supported by National Institutes of Health/National Institute of Child Health and Human Development grant no. K23HD067247.
Conflict of interest: none declared.
- Received for publication March 13, 2014.
- Revision received July 25, 2014.
- Accepted for publication July 29, 2014.