Abstract
Congestive heart failure (CHF) and obesity are common medical conditions that have many complications and an increasing incidence in the United States. Presented here is a case of a disfiguring skin condition that visually highlights the dermatologic consequences of poorly controlled CHF and obesity. This condition will probably become more common as CHF and obesity increase in the US.
A 48-year-old man presented to our clinic complaining of 7 months of worsening, bilateral leg swelling with painful, oozing “water sores.” He also described worsening dyspnea on exertion and 3-pillow orthopnea. His medical history was significant for poorly controlled congestive heart failure (CHF); obesity (body mass index, 43 kg/m2); atrial fibrillation; and diabetes mellitus. He had no significant travel history or family history.
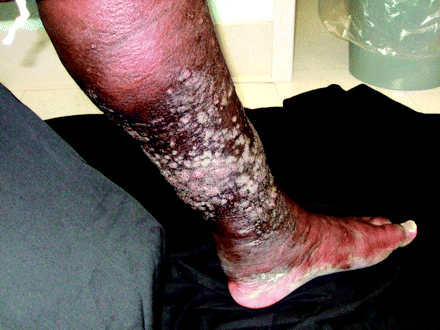
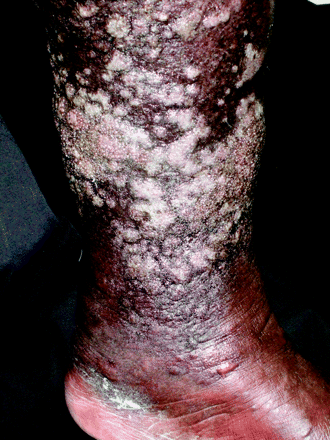
During physical examination vital signs showed mild hypoxia, tachypnea, and a fever of 101.6°F. Jugular venous distension, bibasilar crackles, and an irregular heart rhythm were present. The lower legs revealed significant pitting edema with woody, indurated skin that had a circumferential confluence of weeping plaques with a pebbled, verrucous appearance (see Figures 1, 2, and 3).
Below both knees the patient had tense, woody edema with a circumferential confluence of white- to pink-colored plaques.
The patient's edema was seen especially from the mid-tibia to the ankle.
The confluence of plaques had a verrucous, “cobblestoned” appearance with tightly packed, tense papules. Some papules had weeping ulcerations.
Laboratory evaluation revealed normal complete blood count, cardiac enzymes, metabolic panel, and thyroid studies. Abnormal laboratory values included a C-reactive protein level of 4.1 mg/dL (normal, 0 to 1.0) and brain naturetic peptide level of 342 pg/mL (normal, 0 to 100). Chest radiography showed diffuse pulmonary infiltrates and electrocardiogram showed atrial fibrillation. The patient was admitted for an acute CHF exacerbation and presumed cellulitis. A wound culture grew multiple organisms (Staphylococcus aureus, Serratia marcescens, Citrobacter koseri, Acinetobacter lwoffii) but blood and fungal cultures were negative. A skin punch biopsy was consistent with stasis dermatitis and, based on clinical examination, the patient was diagnosed with elephantiasis nostras verrucosa (ENV) with overlying acute lymphangitis. A conservative therapy was adopted to control his ENV, specifically antibiotics to treat the acute infection and leg elevation and compression stockings to manage the chronic ENV. Although discharged in stable condition, the patient died 3 months later from CHF-related complications.
Discussion
Elephantiasis nostras verrucosa (ENV) is the progressive disfiguring enlargement of a body part caused by recurrent soft tissue bacterial infections in the setting of chronic secondary lymphedema. In 1934 Castellani first used the word “nostras,” meaning “from our region,”1to distinguish elephantiasis nostras from elephantiasis tropica, or filariasis, which is caused by the microscopic, parasitic Wuchereria worms that invade and obstruct the lymphatics. ENV typically appears in gravity-dependent regions, most commonly the lower extremities, but has been reported in the upper extremities, abdominal pannus, buttocks, orbital area, lips, ears, and scrotum.2–4
It is currently unclear which patients with chronic lymphedema progress to ENV. It is hypothesized that the protein-rich fluid that accumulates in the interstitium provokes an inflammatory response that impairs the local immune response and predisposes the patient to soft tissue infections such as erysipelas, cellulitis, and lymphangitis. Inoculation can result from insignificant trauma, poor hygiene, and dry fissured skin.1–7 With each bout of soft-tissue infection the lymphatics become increasingly fibrotic and the affected body part becomes more edematous and enlarged, even after the acute infection has subsided. Meanwhile, the skin undergoes epidermal hyperkeratosis and fibrosis of the dermis and subcutaneous tissues.2,4 In time this produces the characteristic clinical appearance of ENV: an enlarged body part with woody edema and thickened, hyperkeratotic skin that has a cobblestone-like, verrucous appearance. Over time, ulcerations become more common and become another route for infection.
ENV is a form of chronic secondary lymphedema; primary lymphedema is caused by congenital defects in the lymphatic system.5 Secondary lymphedema is the dysfunction of the lymphatic system caused by another primary disease process. Primary infectious etiologies include filarial, staphylococcal, and streptococcal infections, which cause direct inflammation and fibrosis of the lymphatic vessels. Noninfectious etiologies include disruption of the lymphatic system after trauma or surgery; obstruction by malignancy, CHF, and obesity; and lymphatic fibrosis by malignancy, radiation, venous stasis, CHF, obesity, portal hypertension, and scleroderma.2,5 The most common cause of secondary lymphedema in developing nations is filariasis; among industrialized nations it is malignancy.5 Two other causes of secondary lymphedema—CHF and obesity—are on the rise in the United States.
The prevalence of CHF is rising in America and it currently affects 2% of the US population.8 Approximately one quarter of the US population is obese and the prevalence of obesity is rising among all ages, all racial groups, all 50 states, and both sexes.9 As the prevalence of CHF and obesity rise, the complications of these diseases will also become more common; these complications include skin manifestations such as ENV.
CHF and its edema can be the beginning steps that lead to ENV. The decrease in myocardial contractility and cardiac output that is seen in CHF results in an increase in central venous pressure. This pressure is transmitted back to the capillary beds, where fluid leaks into the interstitium. The decreased cardiac output and capillary fluid leak create a functional decrease in intravascular volume, triggering various physiologic mechanisms to increase intravascular volume and thereby raising central venous pressure and worsening the edema.10 Beyond creating proteinaceous lymph, edema can directly overwhelm, impair, and obstruct the lymphatic system and create lymphedema.
Our patient's morbid obesity also played a role in the development of ENV. Obese patients already are at an increased risk for skin infections and impaired skin and soft tissue wound healing. With ENV, it is theorized that excessive adipose tissue can impair lymphatic drainage and lead to the buildup of protein-rich lymphedema and associated fibrosis and inflammation.9 Our patient's CHF and obesity created “the perfect storm” to produce lymphedema, recurrent skin infections, and ultimately ENV.
ENV can be diagnosed clinically with history and physical examination alone, although other tools are available to assist the clinician. The physical examination finding of Stemmer sign—the inability to pinch the skin on the dorsal toes because of skin thickening—is suggestive of lymphedema. Although not necessary for ENV diagnosis, diagnostic studies can assist the clinician in evaluating lymphedema and ruling out other causes. Biopsy can rule out other causes of secondary lymphedema, such as malignancy. Histologically, ENV shows hyperkeratosis of the epidermis, loss of dermal papillae, fibrosis of the dermis and subcutaneous tissues, and widened lymphatic vessels. Lymphoscintigraphy can assist in the diagnosis of lymphedema. Ultrasound can identify filarial adult worms within the lymphatics, although sensitivity is low. Computerized tomography and magnetic resonance imaging can be used to rule out obstructive malignancy.2,11 The differential diagnosis for ENV is broad and is listed in Table 1.
Differential Diagnosis for Elephantiasis Nostras Verrucosa
There currently is no established standard of care for the treatment of ENV. Therapies attempt to correct the underlying cause, maximize the patient's ability to use the affected limb, and prevent further complications.11 Initial conservative treatments include elevation of the affected limb, compression with bandages or stockings, massage, and pneumatic compression devices.1–3,11,13,14 During acute lymphangitis, appropriate antibiotics should be used to treat the infection. When there is no active infection, prophylactic use of antibiotics may be considered.3 There are several reports of success with the use of oral and topical retinoids, such as etretinate and tazarotene, respectively, which decrease epidermal proliferation, fibrogenesis, and inflammation.1–3 If medical measures fail, surgical options are available. These include debridement, lymphatic and lymphovenous anastomosis, lymphatic transplantation and, for severe cases in which the outlook of limb preservation is bleak, amputation.11,14
The prognosis for a patient with ENV is based on the severities of their ENV and their primary condition. Prognosis is improved when the disease process is interrupted early, the patient is motivated to participate in therapy, and there is close follow-up with a primary care physician.2,8,9 Depression, anxiety, and social isolation can develop because of embarrassing and grotesque features. Patients can suffer from disability caused by the loss of use of the affected limb. With further progression, bacterial and fungal super-infections of the skin and underlying bone can develop. The patient may ultimately die from sepsis associated with ENV or from the advancement of their primary condition.2,7
Conclusion
Our patient had no identifiable malignancies, history of radiation therapy, surgical history, history of trauma, history of travel to tropical regions, or family history of similar pathology that would have caused his lymphedema. His advanced CHF and morbid obesity, however, contributed significantly to his lymphedema and resulting ENV. With an increasing prevalence of CHF and obesity in the United States, their complications will also increase in prevalence. ENV is, unfortunately, a disease without well-studied, standardized, or effective treatment, and its prognosis is poor. For the clinician, this case illustrates the importance of early intervention for patients with CHF and obesity to prevent sequelae such as ENV. Ultimately, the best therapy for ENV, as with all complications of CHF and obesity, is primary prevention.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
Disclaimer: The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of Defense.
- Received for publication June 2, 2009.
- Revision received October 9, 2009.
- Accepted for publication October 19, 2009.