Abstract
Background: Upper gastrointestinal complaints are common in primary care. These patients are often referred for evaluation with the use of esophagogastroduodenoscopy. This study examines the feasibility and safety of office-based ultrathin (diameter, 5.9 mm) esophagogastroduodenoscopy (u-EGD) without conscious sedation in a primary care setting.
Methods: This study is a retrospective chart review in a university-based family medicine residency in the southeastern United States. Charts were reviewed for adult outpatients (N = 126) who were referred for further evaluation of heartburn, dyspepsia, or epigastric pain and who elected to undergo u-EGD procedure. We examined the number of patients willing to undergo office-based u-EGD, patient demographics, procedure indications and findings, patient request for oral benzodiazepines, and procedure and recovery times.
Results: Of the 132 patients asked to participate in office-based u-EGD, 126 (95.4%) were willing to undergo this procedure (mean age, 47.6 ± 1.3; 75% women). Of 126 patients, 122 (96.8%) tolerated office-based u-EGD, and 80.6% of patients requested oral anxiolytic medications. Significantly more women than men requested oral anxiolytic medications (84.0% versus 65.6%, respectively; P = .026). The retroflexion maneuver was completed in 120 of 122 (98.4%) patients, and the second portion of duodenum was reached in 122 of 122 (100%) patients. Mean procedure time was 16.9 ± 0.7 minutes, and mean recovery time was 3.8 ± 0.2 minutes. There were no complications reported in this case series.
Conclusions: The majority of patients can tolerate office-based u-EGD without conscious sedation in a primary care setting, but most patients request oral anxiolytic medications. Statistically more women request oral anxiolytic medications than do men.
Primary care physicians are inundated with patients with upper gastrointestinal complaints. In fact, the sixth leading reason for outpatient visits to physician offices is stomach and abdominal pain, cramps, and spasms,1 and gastroesophageal reflux disease accounts for 1% of all office visits to family physicians in the United States.2 Esophagogastroduodenoscopy (EGD) is a common procedure performed in the work-up of patients with upper gastrointestinal complaints.
Today, in the United States, standard or conventional gastroscopy (diameter, 8 to 10 mm) is usually performed in a gastrointestinal or surgical suite under conscious sedation to reduce discomfort and anxiety.3 Over the last decade, an ultrathin (diameter, 5.3 to 5.9 mm) fiberoptic endoscope was developed. Shaker et al4 published the earliest report of unsedated ultrathin EGD (u-EGD) to evaluate the upper gastrointestinal tract. Numerous studies have described sedationless u-EGD5–9; this information has recently been summarized in a systematic review.10
One of the authors (TW) recently evaluated u-EGD performed by a family physician compared with conventional EGD11; however, in this study, all procedures were performed in a hospital-based gastrointestinal suite. To date, no study has evaluated this u-EGD in the outpatient primary care setting. The current study reports our experiences with the feasibility and safety of u-EGD without conscious sedation in a primary care clinic.
Methods
This retrospective chart review examines the medical records of 126 adult, English-speaking, nonemergent outpatients over the age of 18 years, 1 month after completion of an unsedated u-EGD that was performed by or supervised by the author (TW) in a primary care clinic as part of a medical evaluation of upper gastrointestinal complaints. The endoscopist (TW) is a board-certified family medicine physician who has performed EGDs independently for 5 years. The endoscopies in this study were completed between August 2002 and November 2003 using an Olympus XP-160 gastrointestinal videoscope (Olympus America Inc, Melville, NY) (Figures 1 and 2).
Ultrathin endoscope, XP-160
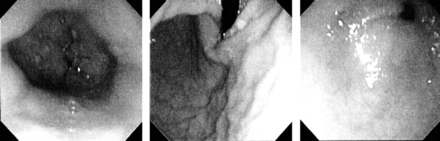
Images taken with XP-160 endoscope. Left: normal distal esophagus. Middle: normal retroflexed view. Right: normal pylorus.
The specifications for the forward-viewing videoscope are an outer diameter of 5.9 mm; an accessory channel of 2.0 mm; a working length of 103 cm; tip deflections of 180° upward, 90° downward, and 100° right and left; and a field of view of 120°. We reviewed the chart records for demographic data, indications for EGD, endoscopic diagnosis, procedure time, recovery time, whether retroflexion and duodenal intubation were completed, and Campylobacter-like organism test results. Biopsies were obtained if clinically indicated. Any complications were also noted.
The primary aim of this study was to demonstrate the safety and feasibility of performing u-EGD without conscious sedation in a primary care clinic. The primary analyses for the study were the descriptive statistics examining endoscopy completion rates and the success of duodenal intubation and retroflexion maneuvers. Subsequent analyses examined whether patient age, gender, or ethnicity influenced completion or complication rates.
Data Analysis
Descriptive statistics were used to evaluate frequencies, percentages, and means of study variables. SPSS version 11 (SPSS Inc., Chicago, IL) was used for all analyses. A χ2 analysis was used to examine differences by sex in anxiolytic medication use during the procedure. A nonparametric Mann-Whitney U test was used to consider differences in procedure times dependent on whether a resident participated in the procedure or not.
Results
Of the 132 patients asked to participate in office-based EGD, 126 (95.4%) were willing to undergo office-based u-EGD without conscious sedation. The six declining patients preferred to have a sedated hospital-based EGD. The mean age of the patients in the study was 47.6 ± 1.3 years; 74.6% were women. The numbers of African American and white persons in the study were roughly equal, 48.4 and 51.6%, respectively. The payor status in this chart review was Medicare (25.4%), Medicaid (15.9%), private insurance (44.4%), and self-pay (14.3%).
The u-EGD procedure was tolerated by 122 (96.8%) of the 126 patients who agreed to an office-based u-EGD without conscious sedation. Retroflexion was performed in 98.4% of the procedures, and duodenal intubation was completed in all them. 100 patients (80.6%) elected to take an anxiolytic medication before the procedure; women were statistically more likely than men to request medication (P = .026). All patients were offered diazepam; however, if patients were taking a different benzodiazepine, they were allowed to take their usual medication. Of the 100 patients, 94 (94.0%) received diazepam, with a mean dose of 10.9 ± 0.3 mg given orally 1 hour before the procedure. Five (5.0%) patients received lorazepam and 1 (1.0%) patient received clonazepam.
Gastritis, hiatal hernia, and esophagitis were documented as EGD findings in 60.7%, 41.8%, and 15.6% of the charts, respectively. These findings are expected given that heartburn and epigastric pain were the most common presenting indications for the EGD examination. A positive CLO test for HP was noted in 20 (16.7%) patients. EGD indications and findings are summarized in Table 1. No complications were noted in this case series.
Indications and Esophagogastroduodenoscopy Findings
The mean procedure time in the study was 16.9 ± 0.7 minutes, and the mean recovery time was 3.8 ± 0.2 minutes. Procedures completed by the author (TW) without resident participation took less average time (13.0 ± 0.8 minutes) than procedures completed with resident participation (20.5 ± 0.8 minutes) (Z = −6.04, P = .000).
Although tolerability of office-based u-EGD was excellent, 4 of 126 (3%) did not tolerate u-EGD without conscious sedation. These patients elected not to continue the procedure within the first 1 to 2 minutes of the procedure. In our series, patients that did not tolerate u-EGD included a 72-year-old white woman, a 37-year-old African American woman, a 33-year-old African American woman, and a 24-year-old white woman. Of the 4 patients who did not tolerate u-EGD, 2 patients had taken an anxiolytic medication.
Discussion
Office-based u-EGD without conscious sedation was almost universally tolerated in this case series (96.8%). Office-based u-EGD without conscious sedation offers many advantages compared with hospital-based EGD with conscious sedation. Patients require less recovery time, patients do not require continuous cardiopulmonary monitoring, only 1 assistant is required, and it is less costly than hospital-based sedated EGD.
The benefit of using an anxiolytic medication in improving tolerability is uncertain. Using an anxiolytic medication negates some of the benefits of performing an unsedated examination (eg, the patient cannot work or drive after the procedure). Future studies should evaluate the use of anxiolytic medications and overall tolerability of u-EGD (eg, pain, anxiety, and gagging). The sample size limited our ability to determine predictors of intolerance to unsedated endoscopy; however, other investigators identified younger age and high pre-endoscopic anxiety levels as predictors of patient intolerance.8,12
New technology comes at an expensive price. Approximate costs of the equipment from Olympus used in this case series is $63,520 (1 XP-160 gastroscope $23,700, CV-160 video processor $19,700, CLV-160 light source $11,120, printer $6,000, and video monitor $3,000). Fujinon and Pentax also offer ultrathin gastroscopes. We successfully billed for office based u-EGD and collected reimbursements for CPT code 43239 (EGD with biopsy) at rates of $150 for Medicare, $170 for Medicaid, and $350-$400 for private insurance.
A comparison of u-EGD with sedated conventional EGD (sc-EGD) is helpful in understanding the differences between these endoscopic procedures. Garcia et al13 reported that sc-EGD costs $512.4 compared with u-EGD that costs $328.6. In Garcia’s study, u-EGD procedure time was 3.5 minutes compared with sc-EGD procedure time of 5 minutes, and u-EGD recovery time was 12 minutes compared with sc-EGD recovery time of 75 minutes.13 A recent systematic review reports up to 40% refusal of u-EGD in United States, which limits the widespread adoption of u-EGD in primary care.10 There are no data to support the contention that u-EGD is safer than sc-EGD; however, limited studies have reported no serious complications with patients who have undergone u-EGD.11,14,15
Our series had a 17% positivity rate for Helicobacter pylori (HP). Another study theorized that the diagnostic yield for detecting HP is not adversely affected by the small biopsy size.16 Other studies have reported on the adequacy of biopsy size for pathologic diagnosis.17,18
Our study had several limitations: there was high percentage of women, 1 endoscopist performed all u-EGD procedures, and it was conducted in a university family medicine residency training site in the southeastern United States. Each of these limitations may decrease the generalizability of our results. This was a retrospective chart review and not an experimental design, which may have led to some selection bias.
A question posed by our prior study was whether patients could tolerate unsedated office-based EGD if performed by a resident in training. This case series demonstrates that although procedure time was significantly longer if residents participated in u-EGD (compared with procedures without resident participation), resident involvement did not adversely affect patient tolerance of the procedure. However, resident training with office-based u-EGD needs to be studied in a systematic manner. Office-based u-EGD seems to be safe and universally tolerated by outpatients in a family medicine center. Adoption of office-based u-EGD by family medicine physicians may provide greater patient access to upper endoscopy.
Notes
This work was presented previously at the American Academy of Family Practice Annual Scientific Assembly; 2003 Oct 1–5; New Orleans, LA.
- Received for publication June 2, 2004.
- Revision received June 2, 2004.