Abstract
Objective: The objective of this study was to determine whether spontaneous peripartum coronary artery dissection (SPCAD) is a cause of acute myocardial infarction in women.
Methods: Patients with SPCAD reported in the recent literature were analyzed to elucidate the clinically relevant characteristics of this condition.
Results: Forty-seven cases of SPCAD are described. Patient characteristics include the following: mean age, 33.5 ± 5.2 years; gravity, 2.7 (95% confidence interval, 1.8–3.5); mean gestational age if prepartum, 32.5 ± 4.2 weeks (range, 23–36 weeks); and mean onset if postpartum, 22.9 ± 26.1 days (range, 3–90 days). Only 17 patients (36%) reported a cardiac risk factor, with the most frequent being smoking. All presented with characteristic ischemic pain; 25% of patients were hemodynamically unstable; and 81% of initial electrocardiograms demonstrated ST-elevation myocardial infarctions. The left coronary artery system was involved 81% of the time. Thirty percent of patients were managed conservatively or with thrombolytic therapy, whereas 34% received emergent percutaneous cardiac intervention and 36% required bypass surgery. There were no maternal deaths. Long-term follow-up revealed good cardiac function in the majority of patients, although 3 women required heart transplantation.
Conclusions: SPCAD can occur weeks before or after delivery and should be considered in women presenting during the peripartum period with acute chest pain.
Spontaneous peripartum coronary artery dissection (SPCAD) is a rare but devastating complication of term pregnancy in women. The majority of the victims are young, have few if any cardiac risk factors, and can dissect days to weeks before or after delivery. They typically incur large myocardial infarctions and present significant challenges to diagnosis and management.
Goals of This Investigation
We recently encountered such a patient and describe her case in detail. In addition, we reviewed the current medical literature relating to this condition and report the general characteristics of this patient population, including presenting signs and symptoms, diagnostic clues, management options, and outcomes.
Case Report
A 25-year old woman presented to the emergency department (ED) with a complaint of pleuritic chest pain. Five days earlier she underwent an uncomplicated cesarean delivery performed at 40 weeks' gestation after attempted elective induction resulted in fetal distress. Her inpatient hospital course was uneventful. After discharge she experienced several brief episodes of sharp chest pain, which were described as mild and transient and resolved spontaneously. On the day of presentation she was breastfeeding her infant when her chest pain recurred and persisted. Initially mild, it became progressively more severe over the course of several hours, with radiation to her back and left shoulder. In addition, she reported worsening shortness of breath and that deep breaths exacerbated her pain. Her symptoms prompted family members to call their local emergency medical services unit for assistance.
The patient arrived via ambulance, having received morphine and aspirin during transport, and immediately was placed in a critical care bay because of severe respiratory distress. She appeared anxious, pale, and diaphoretic and complained of sharp chest pain that was worse with inspiration. During direct questioning, she denied the presence of fever, chills, lightheadedness, cough, hemoptysis, or calf swelling. She had a history of polycystic ovarian syndrome but no history of deep vein thrombosis or pulmonary embolism. She had no known cardiac risk factors. She was not taking any prescribed medications and denied any current or previous drug abuse, including cocaine. She was a nonsmoker.
In addition to a heart rate of 100 beats per minute and a blood pressure of 126/84 mm Hg, her initial vital signs were notable for an irregularly irregular tachycardia and an oxygen saturation of 90% on room air, which was significantly different from the initial prehospital oxygen saturation of 100%, a value obtained approximately 20 minutes earlier at the patient's home. Lung auscultation demonstrated diffuse crackles bilaterally. Heart auscultation revealed an irregular rhythm without murmur or rub. Lower extremity examination was normal without calf tenderness or asymmetry. The remainder of her physical examination was unrevealing.
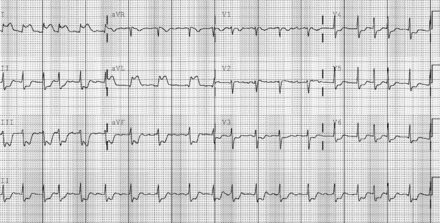
Review of the prehospital 12-lead electrocardiogram (ECG) demonstrated a sinus rhythm with ST elevation in lead I and L, and ST depression in leads II, III and F. These dynamic changes were confirmed with the initial ECG performed in the ED. In addition, her rhythm now demonstrated atrial fibrillation (Figure 1). Serial ECGs performed during her care in the ED exhibited progression of the ST elevation to include leads V1 and V2, consistent with an evolving anterolateral acute myocardial infarction (AMI), and her atrial fibrillation spontaneously converted to a sinus rhythm (Figure 2).
Prehospital electrocardiogram (ECG) demonstrating atrial fibrillation and acute lateral ST-elevation myocardial infarction with reciprocal changes in the inferior leads.
Emergency Department electrocardiogram (ECG) demonstrating return of regular sinus rhythm and evolving antero-spetal ST-elevation myocardial infarction.
Given her rapidly deteriorating condition, the patient was intubated quickly for impending hypoxic respiratory failure. Her chest radiograph demonstrated diffuse alveolar opacities bilaterally, consistent with pulmonary edema. A computed tomography scan of the chest was performed immediately to search for thoracic aortic dissection and pulmonary embolus; the interventional team was notified simultaneously of the need for emergent cardiac catheterization. The patient's thoracic computed tomography scan demonstrated only interstitial edema.
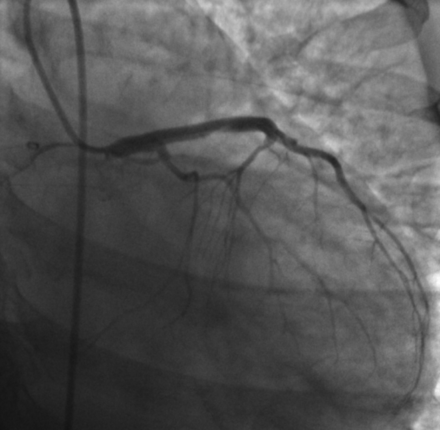
The cause of this young and otherwise healthy woman's acute coronary syndrome was suspected to be spontaneous peripartum coronary artery dissection, and she was transferred emergently to the interventional suite for percutaneous cardiac intervention. Catheterization revealed an extensive dissection involving the left main, left anterior descending, and diagonal coronary arteries (Figure 3). The patient ultimately required surgical revascularization and the placement of a left ventricular assist device. She survived her acute event and subsequently has undergone successful heart transplantation. She now has resumed a relatively normal life with essentially no restrictions.
Emergent cardiac catheterization angiogram demonstrating no flow through the left main, left anterior descending and diagonal coronary ateries.
Methods
A comprehensive MEDLINE search was conducted, identifying published case reports and case series of pregnancy-related spontaneous coronary artery dissection. The search was performed using the terms coronary artery dissection or spontaneous coronary artery dissection and either pregnancy or post*partum for articles published between 1996 and the time of the search (July 2009). Only reports published during this time frame were included to minimize variation from current management. All reports of SPCAD occurring during pregnancy or within 90 days of abortion or delivery were included. Using a standardized data extraction tool, the following elements were collected by a single investigator: age, pregnancy and delivery-related complications, timing of SPCAD, cardiovascular risk factors, presenting symptoms, ECG findings, culprit vessels, interventions, and follow-up cardiac function.
Data were entered into a Microsoft Excel (Microsoft Corp., Redmond, WA) spreadsheet program for cleaning and analysis. Descriptive statistics were used to characterize the study population. Summated data are presented as numbers and percentages. Maine Medical Center's institutional review board designated exempted status to this investigation.
Results
Forty-six published cases of SPCAD were identified.1⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–44 In addition to our sentinel case, these comprise the 47 cases of SPCAD described in this report. Not all data elements were reported in every instance.
Patient demographics, timing of SPCAD, and cardiac risk factors are displayed in Table 1. The mean age of these women was 33.5 ± 5.2 years [95% confidence interval [CI], 31.9–35). The majority reported having previous uneventful pregnancies (mean gravity, 2.7 [95% CI, 1.8–3.5]).
Patient Age, Cardiac Risk Factors, and Timing of Dissection
The presence or absence of cardiac risk factors was documented in 44 cases. Seventeen patients (39%) reported at least one cardiovascular risk factor, with the most frequent being smoking history (23%), family history (16%), hypertension (9%), and lipid disorder (7%). However, only 4 women (9%) had more than one risk factor, whereas 27 (61%) reported no known risk factors. No case reported associated illicit substance use such as cocaine.
Thirty-five women (74%) developed SPCAD during the postpartum period, with a mean interval of 22.9 ± 26.1 days (range, 3–90 days) after delivery. Two of these women experienced SPCAD after aborted pregnancies. For those women developing SPCAD during pregnancy, the mean gestational age was 32.5 ± 4.2 weeks (range, 23–36 weeks).
Patient presenting symptoms, initial ECG changes, identification of the culprit vessel(s), and therapeutic interventions are displayed in Table 2. All patients presented shortly after experiencing chest pain, which was described uniformly with classic ischemic modifiers suggestive of acute coronary syndrome. Associated symptoms and signs were reported in 44 cases and most frequently included dyspnea (34% of patients) and diaphoresis (30% of patients). Seven women (16%) had experienced previous self-limited chest pain.
Patient Complaints, Electrocardiogram (ECG) Changes, Culprit Vessel(s), and Treatment
Ten women (25%) were hemodynamically unstable at the time of presentation; this was defined as hypotension, bradycardia, reported cardiogenic shock, or ventricular fibrillation. Six patients (14%) received advanced cardiac life support interventions and 12 (26%) required emergent intra-aortic balloon pump support.
All women experienced AMI as a result of their SPCAD. Specific initial ECG findings were reported in 42 cases. Of these, 34 (81%) demonstrated ST-elevation myocardial infarctions. Cardiac catheterization, intravenous ultrasound, multidetector computerized tomography, or a combination of these modalities was used to identify the culprit vessel(s). The left coronary artery system (left main, left anterior descending, left circumflex, or a combination of these vessels) was involved 81% of the time (38 cases), whereas the right coronary artery was the culprit vessel in 5 cases (11%). Both left and right coronary artery systems were involved in 3 cases (6%); in one instance the dissection was described as involving only “small branches.”
It is not surprising that the reported treatment strategies used in this cohort of young women with acute coronary syndrome favored procedural over pharmacological interventions. Seventeen women (36%) underwent emergent coronary artery bypass grafting, 16 (34%) received emergent percutaneous intervention, and 14 patients (30%) were managed with either conservative medical or thrombolytic therapy.
Patient outcome data were reported based on patient condition at discharge or at various follow-up appointments. Numerous outcome measures were described, making it difficult to compare cases or identify trends. However, no cases of maternal death were reported, although fetal demise was directly attributed to intrapartum SPCAD in 3 instances (6%). Long-term follow-up, when described, revealed good cardiac function in the majority of patients. Three women eventually underwent successful heart transplantation.
Discussion
Patients younger than age 45 years account for approximately 10% of AMIs in the United States. The majority of this subpopulation are men with known cardiac risk factors and occult atherosclerotic coronary artery disease.1 It is fortunate that peripartum AMI is an uncommon event. A recent US population-based report estimated the incidence of AMI to be 1 in every 16,129 pregnancies, with a mortality rate of approximately 5%.45 Although most of these women are documented to have typical atherosclerotic obstructive coronary artery disease as the cause of their AMI, up to 1 in 4 will prove to have SPCAD.46 The initial diagnostic approach to these young, pregnant women with suspected acute coronary syndrome obviously will be the same, with most undergoing emergent diagnostic coronary artery catheterization, which should differentiate atherosclerotic disease from dissection. However, it is prudent to remember that the ideal management of these patients differs, as described below, and it is important to keep SPCAD on the list of differential diagnoses when dealing with this specific subpopulation of patients.
The incidence of spontaneous coronary artery dissection for the general population with AMI is estimated to be less than 1%.47 However, approximately 25% of pregnant women who experience AMI are found to have SPCAD as the cause, suggesting that hormonal and physiologic factors associated with pregnancy likely play a contributing role. Postulated pathophysiological mechanisms include pregnancy; labor- and delivery-induced increases in cardiac output and arterial sheer forces; alterations in collagen synthesis; increased progesterone and estrogen levels; and alterations in the coagulation and fibrinolysis systems, resulting in a prothrombotic state.4,7,46 The end result is a coronary artery hemorrhagic dissection, with or without an intimal tear, that usually begins within 2 cm of the aortic ostium, extends distally, and compresses the true lumen.
Before the widespread and immediate availability of advanced cardiac services, short-term patient mortality from SPCAD approached 40%.4 It is reassuring that our review of all cases reported during the era of more advanced therapeutic cardiac options failed to identify a single case of maternal mortality. It is possible, however, that publication bias may be contributing to these impressive outcomes.
Our analysis indicates that women with SPCAD can present weeks before delivery (a mean gestational age of 32.5 weeks, with a lower-end range of 23 weeks) or after delivery (a mean of 30 days postpartum, with an upper-end range of 90 days). Therefore, we suggest asking women of childbearing age who present with signs and symptoms suggestive of acute coronary syndrome about their recent obstetric history. In addition, we found that these women are less likely to have predisposing cardiac risk factors and usually present relatively soon after the onset of classic symptoms of ischemic pain. Acute dyspnea and diaphoresis often are experienced as well. One of 4 women will be hemodynamically unstable at the time of arrival at the ED.
The majority of patients with SPCAD will demonstrate electrocardiographic evidence of an ST-segment elevation myocardial infarction, most often in a left coronary artery system distribution. This reflects that more than 80% of these dissections involve the left coronary artery and its major divisions. For the pregnant woman undergoing emergent diagnostic cardiac catheterization, all possible measures should be implemented to protect the fetus from harmful exposure to radiation.
Historically, some women with SPCAD survived with relatively good functional outcomes using medical therapy alone. Most received intravenous heparin without anecdotal reports of adverse effects. However, there also is anecdotal and theoretical evidence to suggest that thrombolytics may potentiate the hemorrhagic dissection.48,49 In addition, thrombolytics are relatively contraindicated during pregnancy (class C, with no reliable studies reported).7 It is important to weigh the potential benefits of thrombolytic therapy with the risks of harm in every specific circumstance. It has been recommended that thrombolytics should be avoided for at least 2 weeks after delivery of the fetus.22
The currently preferred management option for SPCAD is percutaneous coronary intervention if the dissection is anatomically amenable to stenting. However, given the nature of these lesions, coronary artery bypass graft surgery is often required. Finally, it should be emphasized that women with prepartum SPCAD should be managed by a multidisciplinary team, including an obstetric consultant.12 These women seem to have a high likelihood of surviving their event with near-normal long-term cardiac function.
A potential limitation of this review should be mentioned. There is the possibility of publication bias, with cases demonstrating favorable clinical outcomes being preferentially submitted for consideration. However, reviewing the medical literature (mainly case reports) before our cutoff of 1996 suggests that the maternal and fetal mortality from this condition was not insignificant. Therefore, the impressively favorable outcomes observed in our review (no reported maternal mortality) might well represent the impact of recent advances in the overall care of patients experiencing acute coronary syndromes coupled with the general health of this selected patient population.
Conclusion
It is important for clinicians to consider SPCAD in women of childbearing age who present with signs and symptoms consistent with an acute coronary syndrome. Obtaining a recent obstetric history will identify the possibility of this condition. SPCAD can mimic other more common pregnancy-related conditions such as pulmonary embolism, resulting in a delay in diagnosis and definitive management.
Notes
-
This article was externally peer reviewed.
-
Funding: none.
-
Conflict of interest: none declared.
- Received for publication January 22, 2012.
- Revision received June 12, 2012.
- Accepted for publication June 19, 2012.