Abstract
Women with severe or uncontrolled asthma are at higher risk for pregnancy complications and adverse fetal outcomes than women with well-controlled asthma. Recent evidence-based guidelines have concluded that it is safer for pregnant women with asthma to be treated pharmacologically than to continue to have asthma symptoms and exacerbations. According to the Asthma and Pregnancy Working Group (APWG) of the National Asthma Education and Prevention Program, optimal treatment of asthma during pregnancy includes treatment of comorbid allergic rhinitis (AR), which can trigger or aggravate asthma symptoms. In general, treatment of both asthma and AR during pregnancy should follow the same stepwise approach that is used in the general population. This article presents the specific recommendations from the most recent APWG report and from other systematic reviews about which asthma and allergic rhinitis drugs should be preferred during pregnancy. Of the corticosteroids, budesonide has the most data and is listed as Pregnancy Category B (no evidence of risk in humans). Other inhaled and intranasal corticosteroids have less data and are listed as Pregnancy Category C but may be continued during pregnancy if the patient's asthma was well controlled with the medication before pregnancy. Family physicians should help their patients control allergic rhinitis and asthma during pregnancy, encouraging adherence to needed medications.
Asthma is potentially the most common chronic medical condition to complicate pregnancy.1 It was estimated that 3.7% to 8.4% of pregnant women in the United States were affected by the disease between 1997 and 2001.2 Evidence-based guidelines from two national professional societies, the British Thoracic Society and the APWG of the National Asthma Education and Prevention Program (NAEPP), state that pregnant women with asthma should receive optimal treatment.1,3 APWG further specifies that the goals of treatment are to maintain asthma control for the health and quality of life of the mother, as well as for normal fetal development.1 Both groups have concluded from systematic reviews of the literature that asthma treatment is a much safer option for pregnant women than nontreatment of continuing asthma symptoms.1,3
According to APWG, optimal treatment of asthma includes treatment of comorbid AR.1 Up to 80% of adults with asthma also have AR, and 20% to 50% of patients with AR have coexisting asthma.4,5 Due to the increased occurrence of nasal symptoms in pregnancy,6,7 AR may be overlooked. Previously recognized AR should continue to be treated and the evaluation of new onset rhinitis should include consideration of AR.
Consensus is building that asthma and AR are a single airway disease. Prospective data from multiple studies show that AR is an independent risk factor for adult-onset asthma.8–11 In fact, in a recent study, monotherapy with intranasal beclomethasone was as effective as inhaled beclomethasone.12
A secondary analysis of the Kaiser-Permanente Prospective Study of Asthma During Pregnancy indicated that improvement or worsening of nasal symptoms was associated with improvement or worsening of asthma, respectively.13 The researchers concluded that the course of AR during pregnancy may help to predict the asthma course, and that aggressive treatment of worsening AR in pregnant women may improve asthma control.13 This review presents the latest conclusions of evidence-based reports and other relevant data to guide clinicians in caring for pregnant patients with asthma and comorbid AR.
Effects of Asthma and AR on Pregnancy
Uncontrolled AR in pregnant women can trigger asthma or aggravate comorbid asthma (see www.nhlbi.nih.gov/health/prof/lung/asthma/astpreg.txt).14,15 Acute asthma episodes are a particular concern in pregnant women because they can significantly decrease fetal oxygenation.16,17 Severe or uncontrolled asthma is associated with adverse maternal complications (including preeclampsia, vaginal hemorrhage, and complicated labor) and adverse fetal outcomes (including perinatal mortality, intrauterine growth restriction, preterm birth, low birth weight, and neonatal hypoxia).1,14,18 Conversely, women with well-controlled asthma and appropriate treatment have little or no increased risk of adverse maternal or fetal outcomes.1,14
Effects of Pregnancy on Asthma and AR
The “rule of thirds” applies to pregnant women with pre-existing asthma: approximately one third show worsening of asthma symptoms, one third experience improvement, and one third have no change in their asthma.13,19 Similarly, pre-existing symptoms of chronic AR may remain unchanged, improve, or worsen.20
A potentially serious problem during pregnancy is patient nonadherence with pharmacologic treatment, due to fears about risk to the fetus. In a national online survey, conducted in January 2003, Harris International asked 501 women with asthma, aged 18 to 44 years and enrolled in managed care, about their attitudes toward medication use during pregnancy.21 Many of the women said that they expected to feel torn between their own health and the health of their unborn baby.21 Of the women currently using any type of asthma medication, 14% said that they would possibly discontinue its use during pregnancy, and 15% said that they would definitely discontinue its use.21 Of the respondents who had been pregnant at some point in the past while using asthma medication, 39% reported having discontinued or reduced it, and one third of that subset did so without consulting a physician.21 Similarly, 40% of 211 pregnant women making their first visit to an asthma educator acknowledged nonadherence to inhaled corticosteroids (ICS).22 Since the women surveyed were motivated enough to attend asthma education, the attitudes of the study population may not reflect the attitudes of the general population; it is likely that the actual proportion of pregnant women who are nonadherent with asthma medication is even higher. A recent analysis of a managed care organization's database showed that among 334 women using asthma medication before pregnancy, claims for short-acting beta2-adrenergic agonists (SABAs) declined by 52% during pregnancy, and claims for ICS declined by 36%.23 The number of these women who had an asthma-related emergency department visit was 21% higher during pregnancy than it had been previously.23 Adherence data are not available for pregnant women using AR medication.
Adjustments to Standard Care of Asthma and AR During Pregnancy
APWG recommends monthly monitoring of asthma symptoms during pregnancy,1 and family physicians who do not have extensive experience in asthma care should comanage patients with a primary care physician who does have experience or an asthma specialist. APWG also advises serial ultrasound examinations, starting at 32 weeks’ gestation, for pregnant women whose asthma is not well controlled and for those who have severe or moderately severe symptoms.1 Another difference in caring for pregnant patients is that possible allergies should be evaluated by in vitro testing such as RAST or Immunocap and not skin testing, because of the chance of inducing anaphylaxis.24
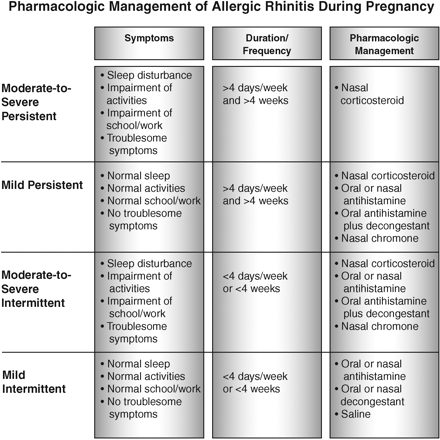
Until recently, AR was categorized as seasonal or perennial. Now, an international consensus panel, ARIA (Allergic Rhinitis and Its Impact on Asthma), has developed a classification similar to the classification of asthma, and has recommended a stepwise approach to AR treatment based on the severity and duration of symptoms (Figure 1). This approach is also advisable when caring for pregnant patients.25
Pharmacologic management of AR during pregnancy.53 Adapted with permission from Knudtson M. Once-daily intranasal corticosteroids for allergic rhinitis, examining treatment issues. Adv Nurse Pract 2006;14:57–60.
Therapy Options
Allergen and trigger avoidance should be an integral part of asthma and AR treatment during pregnancy, especially since it may reduce the need for medication.1,25,26 In particular, pregnant patients should receive advice and medical support for smoking cessation, if needed.,1,3,16 The American Academy of Allergy, Asthma and Immunology has compiled a list of environmental control measures that reduce allergen exposure. This information is available online at www.theallergyreport.com.27
Immunotherapy may be continued during pregnancy, but the dose should not be increased and may be reduced. Initiation of immunotherapy during pregnancy is generally not warranted because of the small, but real, risk of systemic reactions.28,29
If asthma and/or AR symptoms are not controlled with conservative measures, drug therapy is necessary. When prescribing for pregnant patients, it is important to consider both the risk of toxicity to the fetus and the risk to the mother and the fetus of not treating asthma and AR adequately.29
To assist physicians in assessing the risk of toxicity to the fetus, the US Food and Drug Administration (FDA) categorizes drugs as A, B, C, D, or X (Table 1), based on the level of animal and human evidence that supports or refutes an association between use of the drug and congenital anomalies.30 No medications for the management of asthma or AR have been rated Pregnancy Category A (controlled studies show no risk to the fetus). As shown in Tables 2 and 3, some drugs used to treat asthma or AR are rated Pregnancy Category B (no evidence of risk in humans), but most are Pregnancy Category C (risk cannot be ruled out). Pregnancy Category C generally reflects a lack of studies. Drugs with evidence of potential harm are ranked Pregnancy Category D.
FDA Pregnancy Risk Categories54
However, there are limitations to the FDA rating system. The requirement for a well-controlled study in pregnant women, which may not always be ethically or financially feasible, limits the number of drugs that will be rated Category A, giving the impression that only very few drugs have no risk. The ratings also make no distinction of level of risk between trimesters, eg, a medication may be safe to use in the first trimester, but not in the second or third.31
In addition to the FDA rating system, the Teratogen Information System (TERIS), also provides a rating system; “based on the reproducibility, consistency, and biological plausibility of available clinical, epidemiologic, and experimental data” (see the TERIS preamble online http://depts.washington.edu/∼terisweb/teris/preamble.htm) (Tables 2 and 3). The TERIS rating gives the magnitude of teratogenic risk to a child born after exposure during gestation and the quality and quantity of data on which the risk estimate is based.32
Safety of Asthma Drug Classes During Pregnancy SABAs
APWG recommends SABAs as quick-relief medication for pregnant women with mild intermittent asthma.1 Because of the greater amount of data available, albuterol is preferred over other agents (strength of recommendation (SOR)-A).1 Albuterol and the other SABAs commonly used for treatment of asthma are rated Pregnancy Category C.
Physicians should monitor patients’ SABA use. Long-term controller medication may be indicated for pregnant women with intermittent asthma who use their SABA more than 2 times per week and for those with persistent asthma who increase their SABA use to more than 2 to 4 times per week.1
Inhaled Corticosteroids
ICS are the preferred long-term control medications for all pregnant women with asthma, except those with mild intermittent symptoms.1 APWG concluded from its most recent systematic review that ICS therapy during pregnancy improves lung function and reduces the risk of acute exacerbations.1 No studies have documented an increased risk of congenital malformations or adverse perinatal outcomes in women using ICSs.1
APWG cites budesonide as the first-choice ICS during pregnancy because the preponderance of gestational studies involved that agent and the data are reassuring (SOR-A); the earlier recommendations from APWG for beclomethasone were due to the amount of clinical experience with that compound at that time.1 Budesonide has received a Pregnancy Category B rating from the FDA; all other ICS approved for asthma treatment are rated Pregnancy Category C. The Pregnancy Category B rating for budesonide is based on examination of the inhaled and intranasal formulations in population-based, prospective studies that reviewed data from 3 registries covering approximately 99% of all pregnancies in Sweden from 1995 to 2001 (see www.fda.gov/medwatch/SAFETY/2004/jul_PI/Rhinocort_PI.pdf).33,34 Published analyses of the registry have shown that use of inhaled budesonide early in pregnancy does not increase the risk of congenital malformations35 or other adverse pregnancy outcomes.36
No data suggest that other ICS are unsafe during pregnancy, and APWG advises that patients whose asthma is well controlled with another ICS prepregnancy may continue taking that drug.1
Long-Acting Beta2-Adrenergic Agonists (LABAs)
For severe persistent asthma during pregnancy, APWG recommends the combination of a LABA and an ICS as the preferred therapy.37 This combination is also one of the preferred therapies for moderate persistent asthma.37 Very few data are available, but salmeterol has been suggested to be preferable to formoterol because it has been used longer in the United States (SOR-B).37 Except for prolonged retention in the lungs, the pharmacological and toxicological profiles of the LABAs are similar to those of the SABAs.1 Both salmeterol and formoterol are rated Pregnancy Category C. Recent warnings and the recommendation for use of LABAs only after moderate doses of ICS have failed to produce control should also be applied to pregnant women.38
Oral Corticosteroids (OCSs)
APWG advises the addition of an OCS to a high-dose ICS for pregnant women who have uncontrolled severe, persistent asthma.1 Its latest review determined that OCS use during pregnancy, especially the first trimester, is associated with an increased risk of isolated cleft lip, with or without cleft palate (estimated excess risk 0.2% to 0.3%, compared with the general population). Other complications linked to OCS use during pregnancy include preeclampsia, preterm delivery, and low birth weight. Unfortunately, given the scanty amount of data, APWG reviewers could not distinguish the effects of OCSs on maternal and fetal health from the effects of severe or uncontrolled asthma.1 For now, they conclude, it is better to avoid the known risks of poorly controlled asthma than to avoid the uncertain risks of OCSs (SOR-B).1 OCSs are rated Pregnancy Category C. The primary care physician should strongly consider seeking consultation in patients with daily asthma symptoms not controlled with a higher-dose ICS or combined ICS and LABA.
Cromones
Cromones are not a preferred therapy because of their limited effectiveness compared with ICS (SOR-A).1 Both cromolyn and nedocromil are rated Pregnancy Category B, based primarily on safety in animal reproduction studies.18 In the few human studies of cromolyn, no increased risk of adverse pregnancy outcomes has been detected.1
Leukotriene Modifiers
Although minimal data are available, APWG does not recommend discontinuation of the leukotriene receptor antagonists (LTRAs), montelukast and zafirlukast, as therapy for pregnant women whose asthma was controlled with one of these drugs before pregnancy (SOR-B).1 Montelukast and zafirlukast carry the Pregnancy Category B rating, based primarily on animal data.18
In contrast, animal reproduction studies have demonstrated adverse outcomes with zileuton, a leukotriene lipoxygenase inhibitor, including a 2.5% risk of cleft palate in rabbits at a dose analogous to the maximum recommended human daily dose.18 Zileuton is rated Pregnancy Category C.
For pregnant women with mild persistent asthma, APWG accepts the substitution of an LTRA other than Zileuton for low-dose ICS therapy. For those with moderate, persistent asthma, an LTRA may be added to the ICS.1
Theophylline
At recommended doses (to a serum concentration of 5 to 12 μg/mL), theophylline is an alternative to the preferred treatment of a low-dose ICS for pregnant patients with mild, persistent asthma, according to APWG. Theophylline may also be added to ICS therapy for the treatment of moderate to severe persistent asthma during pregnancy (SOR-A).1 However, treatment with theophylline during pregnancy requires regular (at least monthly) monitoring of blood levels.
In animals, high-dose theophylline has been associated with adverse pregnancy outcomes, including congenital malformations.1 In humans, the drug does not seem to increase the risk of congenital malformations, and data conflict about whether theophylline exposure is associated with an increased risk of preterm birth and preeclampsia.18 Theophylline is rated Pregnancy Category C because of the demonstrated risk in animal studies and the lack of well-designed human studies.18
Safety of AR Drug Classes During Pregnancy Antihistamines
In 1993, the NAEPP Working Group on Asthma and Pregnancy (the predecessor of APWG) recommended the first-generation agents chlorpheniramine and tripelennamine as the antihistamines of choice during pregnancy, based on duration of availability as well as reassuring animal and human data.14 However, the ARIA guidelines, published in 2001, conclude that the older antihistamines have an overall unfavorable risk/benefit ratio, even in the nonpregnant population, because of their poor selectivity and their sedative and anticholinergic effects. ARIA recommends that where possible, first-generation antihistamines should no longer be prescribed as AR therapy (SOR-C).29 In general, second-generation antihistamines are more potent, have a longer duration of action, and produce minimal sedation.29
In a joint position statement published in 2000, the American College of Obstetricians and Gynecologists and the American College of Allergy, Asthma and Immunology (ACOG-ACAAI) recommended consideration of cetirizine and loratadine, preferably after the first trimester, for pregnant women who need maximal topical therapy and cannot tolerate chlorpheniramine or tripelennamine.39 ACOG-ACAAI based this statement on reassuring animal data for these second-generation antihistamines, which carry a Pregnancy B rating, and the fact that they are associated with fewer anticholinergic and sedative effects (SOR-B).39
APWG does not mention first-generation antihistamines and recommends cetirizine and loratadine as the second-generation antihistamines of choice for treatment of asthma with comorbid AR.1 A review published in 2005, focusing on the treatment of AR rather than asthma, suggests there is insufficient evidence to support first-line use of cetirizine and loratadine during pregnancy40 and recommends first considering chlorpheniramine, tripelennamine, or hydroxyzine if an antihistamine is needed during pregnancy (SOR-B).40
Physicians must decide on a case-by-case basis whether to select one of the older, better-studied antihistamines, thought to be safe during pregnancy, or a newer agent that has less adverse impact on quality of life but is less well studied in pregnancy.41 The dilemma can often be averted by prescribing an intranasal steroid (INS) or cromolyn instead of an oral antihistamine.41
Intranasal Corticosteroids
In their recent systematic review of AR drug therapy during pregnancy, Gilbert et al40 recommend INS as first-line treatment over oral antihistamines and cromones, based on efficacy. A number of other evidence-based reviews have concluded that INS are the most effective medication for overall AR symptom control and that there are no differences among them in safety or efficacy (SOR-A).28,29,42–45 In addition, treatment of AR, in particular with INS, has been shown to improve asthma symptoms.4,14,46,47
Intranasal budesonide was recently upgraded to Pregnancy Category B; the other INS approved for treatment of AR remain Pregnancy Category C. The upgrade was based on the Swedish birth registry studies mentioned above (in the section on ICS), plus an analysis of the registries that found no significant association between congenital cardiovascular defects and use of intranasal budesonide during early pregnancy.48 A recent systematic review of maternal exposure to inhaled or intranasal budesonide concluded that while data about pregnancy outcomes are limited, the safety profile of intranasal budesonide is at least comparable to that of the inhaled formulation (SOR-A).18
Pregnant patients often need education and reassurance about the safety of intranasal “steroids.” Evidence-based reports have concluded that INS are not associated with significant systemic side effects.28,29 Furthermore, ACOG-ACAAI and APWG have affirmed that INS have a low risk of systemic effects when used at recommended doses.1,39 In the one published prospective human study of INS use during pregnancy, 8 weeks of intranasal fluticasone did not have any deleterious effects on fetal growth or pregnancy outcome.49 There are no currently published studies examining pregnant patients treated with both INS and ICS at the same time.
Decongestants
Decongestants do not improve nasal itching, sneezing, or rhinorrhea, but they are very effective against nasal obstruction.29,43 Short-term use of intranasal decongestants such as oxymetazoline (Pregnancy Category C) can be helpful for nasal congestion that interferes with sleep, but pregnant women should reserve their use until after the first trimester and avoid them during labor (SOR-B).24 Some experts recommend completely avoiding intranasal decongestants during pregnancy, even after the first trimester, due to the lack of sufficient human data (SOR-B).25
ARIA advises that due to the risk of rhinitis medicamentosa, intranasal decongestants should not be used (even by nonpregnant patients) for more than 9 days.31 Pregnant women often favor topical over-the-counter medications over prescription medications, believing them to be safer.24 Physicians should specifically ask about the duration of self-treatment with nasal sprays and explain the risks.50
Case-control studies have linked first-trimester use of pseudoephedrine51,52 (Pregnancy Category C) and phenylpropanolamine51 (recently withdrawn from the US market) with gastroschisis (an abdominal wall defect in which the intestines protrude outside the fetus).51,52 For this reason, ACOG-ACAAI recommends avoiding oral decongestants during the first trimester unless a compelling benefit is expected (SOR-B).39 ARIA suggests avoiding pseudoephedrine during pregnancy and using other decongestants with caution (SOR-B).29 APWG notes that, if a nasal decongestant is indicated in early pregnancy, an external nasal dilator strip, short-term topical oxymetazoline, or an INS can be considered before an oral decongestant.1 Physicians should caution pregnant patients that many over-the-counter cold and allergy remedies contain pseudoephedrine.
Cromones
Intranasal cromones are no longer considered preferred therapies for pregnant women with AR. A recent systematic review of AR treatment during pregnancy categorizes intranasal beclomethasone and intranasal budesonide along with cromolyn as being low-risk, and it recommends INS over cromones as first-line therapy (SOR-A).40
Nedocromil is not recommended over nasal cromolyn or an INS during pregnancy due to the lack of human safety data, however, if a patient had responded well to such treatment previously, then the benefit/risk ratio may favor its continuation (SOR-B).39
Leukotriene Modifiers
As discussed above in the section on asthma drugs, the safety of leukotriene modifiers during pregnancy is not well established. They are not mentioned in the most recent systematic review of AR drug therapy during pregnancy.40
Patient Counseling and Monitoring
If possible, women with asthma should be counseled before pregnancy about the importance and safety of continuing their asthma and AR medications. Once a woman with asthma and/or AR becomes pregnant, she should be given a management plan that indicates when to contact her clinician for medication changes, especially if symptoms worsen. Regardless of whether asthma symptoms improve, remain unchanged, or worsen in early pregnancy, they can be expected to decrease substantially in the last month of gestation. In most women, asthma reverts to the prepregnancy course during the first 3 months postpartum.19 At all stages of pregnancy, it is imperative that adherence to the medications be emphasized. The full benefit of the medications will not be achieved unless taken properly.
A physician comfortable with asthma care should question each pregnant woman with asthma about asthma symptoms. Spirometry or peak flow testing can be helpful in assessing the adequacy of treatment.1,16 APWG recommends spirometry at the time of initial assessment and measurement of peak expiratory flow at follow-up outpatient visits. For patients with moderate to severe asthma, daily peak expiratory flow monitoring is recommended, which may also assist in home monitoring.1
As with all medication therapy during pregnancy, patients should understand the risks and benefits.24,39,41
Conclusions
Adequate control of asthma and AR is necessary during pregnancy to ensure the best maternal and fetal outcomes. Given low adherence, often based on perceived safety risks by patients, physicians will usually need to carefully explain the important and substantial benefits and small risks of medication for good asthma and AR control during pregnancy.
Acknowledgments
The authors acknowledge John E. Fincke, PhD, from Tri-Med Communications (Media, PA) for providing medical writing support, funded by AstraZeneca LP.
Notes
This article was externally peer-reviewed.
Funding: This work was funded by a National Heart, Lung, and Blood Institute/Schering Plough research study on asthma (to BY). No payment for the writing of this article was received from AstraZeneca.
Conflict of interest: BY serves on the respiratory disease advisory councils for Schering Plough, Merck, Boehringer Ingleheim, and AstraZeneca. MK serves on the Speakers Bureau or advisory board for Aventis, Pfizer, and Proctor and Gamble.
- Received for publication August 21, 2006.
- Revision received November 28, 2006.
- Accepted for publication December 5, 2006.