Abstract
Despite recent advances in the assessment, risk stratification, and treatment of acute pulmonary embolism (PE), it remains a leading cause of cardiovascular morbidity and mortality in the United States each year. Patient presentation and prognosis are heterogeneous, and a variety of diagnostic and therapeutic instruments have arisen to assist in providing patients with the appropriate level of care and aggressiveness of approach. Fortunately, a growing number of institutions now have pulmonary embolism response teams (PERT) that urgently assist with risk assessment and management of patients with massive and sub-massive PE. In service of providers at the point of contact with acute PE, this review aims to summarize the data pertinent to rapid risk assessment and the interpretation of diagnostics used to that end. The role of PERT and the indications for systemic fibrinolysis and invasive therapies are also discussed.
- Cardiology
- Clinical Decision-Making
- Echocardiography
- Fibrinolysis
- Patient Care Team
- Pulmonary Embolism
- Pulmonary Medicine
- Radiology
- Risk Assessment
- Thrombolytic Therapy
- Venous Thromboembolism
Despite recent advances in the assessment, risk stratification, and treatment of acute pulmonary embolism (PE), it remains a leading cause of cardiovascular morbidity and mortality, resulting in over 350 000 hospitalizations and over 100 000 deaths in the United States each year.1,2 The indirect costs of PE and venous thromboembolic events are also considerable, both concerning increased rates of long-term disability and decreased quality of life.3 The distribution of these insults among patients with PE is heterogenous in the extreme. Treatment options include outpatient management with oral anticoagulation, administration of high-risk fibrinolytic therapy, catheter-directed thrombolysis, or cardiothoracic surgery.4 Finally, as our understanding of acute PE and its chronic sequelae has deepened, a carefully selected intermediate-risk group of patients has been identified who experience morbidity and mortality benefits following targeted invasive therapies.5⇓–7
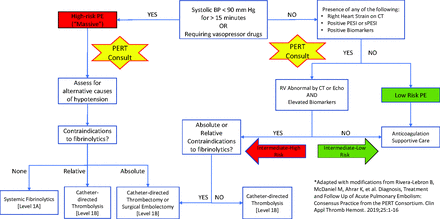
It follows that clinical decision-making in acute PE is complex, and the presence of systemic fibrinolytics in the decision-tree guarantees that the stakes are high. Fortunately, PE has remained an area of active research in recent years, and a variety of tools are now available to assist providers on the front lines in accurately distinguishing between these presentations. The proliferation of pulmonary embolism response teams (PERT) at tertiary centers throughout the United States provides a rapid subspecialty response in intermediate- and high-risk cases.8 The present article aims to examine the following recommendations: (a) furnish providers at the point of contact with acute PE with the means to perform an accurate risk assessment, (b) review the data directing the use of systemic fibrinolysis in high-risk patients, and (c) provide an overview of the PERT process, with emphasis on how patients are selected for advanced therapies, highlighting the crucial role of providers at the point-of-contact in re-stratifying patients as “intermediate-low” and “intermediate-high” risk.
Definitions
The American Heart Association (AHA) divides patients presenting with acute PE into 3 similar groups according to their risk of near-term mortality.11 The majority of this risk is borne by the 5 to 10% of patients who present with sustained hypotension.9,10 These are the patients with “massive” or “high-risk” PE, which the AHA defines as acute PE associated with any of the following 3 features: (1) hypotension defined as a systolic blood pressure <90 mmHg sustained for at least 15 minutes, (2) a drop of >40 mmHg in systolic blood pressure for at least 15 minutes, or (3) a vasopressor requirement.11,12 Massive PE is associated with in-hospital and 90-day mortality rates approaching 25% and 50%, respectively, when treated with anticoagulation alone.9,13,14 Early and aggressive treatment with systemic fibrinolytics has been shown to halve the near-term mortality associated with massive PE, which translates to an absolute reduction in mortality of over 10%.14
While mortality among normotensive patients with PE is much lower, an intermediate-risk subgroup is characterized by the presence of right heart strain or myocardial necrosis. PE associated with right ventricular (RV) injury or dysfunction is designated “submassive” and is associated with a 30-day mortality of 3% to 5% when treated with anticoagulation alone.15,16 There is little data regarding long-term sequelae specific to submassive PE. However, dyspnea and chronic thromboembolic pulmonary hypertension (CTEPH) were recently reported at 3-year follow-up in 33% and 2.7% of the PEITHO trial subjects, respectively.17 CTEPH is uncommon, complicating less than 4% of cases.17,18 Outcomes data regarding the use of systemic thrombolytics in this population have been inconsistent, with 1 high-quality randomized controlled trial finding no benefit due to increased major bleeding events.16 A subsequent meta-analysis suggested evidence of benefit with careful patient selection.15 Both massive and submassive PE demonstrate improved hemodynamics and right ventricular function in the days after therapy, though long-term outcomes remain uncertain.19,20
Finally, the remaining 50% to 60% of patients with PE comprise the low-risk group, which is characterized by the absence of hemodynamic instability, RV injury, or RV dysfunction. Their 30-day mortality due to PE is less than 1%, long-term sequelae are typically absent, and outpatient treatment with oral anticoagulants is often appropriate.21
As recently as a decade ago, 2 notable problems remained unaddressed by this scheme. First, systemic fibrinolytic therapy is associated with an absolute excess risk of major bleeding of 6% to 12% and with intracranial bleeding rates of 0.5 % to 3%.14,16,22,23 In patients with contraindications to fibrinolysis the risks may be much higher, and yet 40% of patients in 1 high-quality study received this therapy despite their presence.13 This finding underscores both the high prevalence of risk factors for bleeding in this patient population and the desperate position in which the patient and physician often found themselves when faced with massive PE. Second, though these high rates of major bleeding make systemic fibrinolytic therapy a dangerous option for routine use in intermediate-risk patients, treatment with anticoagulation alone results in unacceptable morbidity and mortality for many of these patients.
The PERT Process
The first Pulmonary Embolism Response Team (PERT) was developed in 2012 to fill these practice gaps. Armed with a variety of surgical and catheter-based interventions as well as multimodality imaging, PERTs provide a rapid response to hemodynamically significant acute PE, streamlining multidisciplinary consultation and resource mobilization for patients with massive or submassive PE in the inpatient or emergency department settings.24 PERT programs now function in over 100 hospitals in the United States, where they consist of subspecialists from interventional cardiology, interventional radiology, critical care, cardiothoracic surgery, and vascular medicine, among others.8
While subspecialty participation and PERT protocols differ somewhat between institutions, the PERT Consortium has provided standardization by way of diagnostic and treatment algorithms.5 In general, PERT activation occurs after acute PE is diagnosed by computed tomography angiography (CTA) in patients suspected to be at intermediate or high risk of near-term mortality based on clinical presentation, elevated cardiac biomarkers, or evidence of right heart strain on echocardiography or CTA. The designated responder makes a prompt and focused evaluation of the patient, with the goals of risk stratifying the patient for 30-day mortality while assessing for contraindications to first-line therapy. If interventional therapy is warranted, selection of the optimal modality is made in the multidisciplinary discussion, and recommendations are given regarding outstanding diagnostics as well as anticoagulation, pressor support, and the necessary level of care.5
The PERT Assessment
Acute PE threatens life by imposing an intolerable pressure-load on the right ventricle (RV). When large enough, the pulmonary embolus serves both as a mechanical outflow obstruction and a cause of microvascular vasoconstriction in the pulmonary circuit, impairing cardiac output and gas exchange, and resulting in the hypoxia and circulatory collapse that are characteristic of this state. It follows that the PERT responder's first and most important task is to generate a comprehensive assessment of the risk of catastrophic RV failure, stratifying them as having a high, intermediate, or low risk of 30-day mortality from this cause by direct and indirect assessment of RV health and function.
The most critical prognosticator is the presence or absence of sustained systemic hypotension, as it is the most emergent finding in acute PE save for sudden cardiac death and is associated with a 3-fold increase in mortality versus normotensive, symptomatic PE.22,25 In hypotensive patients with acute PE, causality must be established before the diagnosis of massive PE is made, and alternative causes of hypotension must be excluded. Findings of RV dilation and dysfunction on echocardiography are supportive, as are the presence of saddle embolus or large thrombus burden on the diagnostic CTPA. Once the diagnosis of massive PE is confirmed, a rapid assessment for contraindications to systemic fibrinolysis is the last critical step before definitive therapy.
In normotensive patients, assessment of RV health often begins with a study that has already been performed, as several CTPA findings are robustly predictive of adverse short-term outcomes. These include abnormal position of the interventricular septum, reflux of contrast into the inferior vena cava, and indices of RV enlargement. In a prospective study of CTPA for risk assessment in patients with acute PE, a ratio of RV-to-left ventricular (LV) diameters greater than 1.0 was found to have a sensitivity of 85% for 30-day mortality due to PE, though specificity was poor.26 A ratio of RV-to-LV 3 Days volumes greater than 1.2 was equally sensitive for this outcome. The most specific finding, reflux of contrast into the inferior vena cava (86%), is seldom seen in other hypotensive states and may assist the clinician in excluding alternative causes of hemodynamic compromise. In patients with a small thrombus burden on CTPA (ie, subsegmental PE), the absence of these findings is often sufficient to stratify the patient as low risk.
The PERT responder's assessment continues with clinical data gathering, and the estimation of risk by either of 2 well-validated risk scores, the Pulmonary Embolism Severity Index (PESI) and the Simplified PESI. The PESI relies on the assessment of 11 clinical variables, including age, history of cancer, and vital signs to categorize patients into 5 risk classes.27 Developed in 2005, the PESI is a highly sensitive instrument for prediction of 30-day mortality.27,28 The Simplified PESI was derived by subjecting each of the clinical criteria to a univariate logistic regression and removing those that did not correlate significantly with death at 30 days.29 When scores corresponding to risk classes I and II are considered as negative results (ie, the “reference range”), both indices boast sensitivities exceeding 90% and negative predictive values exceeding 98%. Taken in conjunction with negative echocardiographic, serum, and CT indices of RV injury, a low-risk PESI or Simplified PESI score furnishes a compelling argument for medical therapy (Table 1).
Original and Simplified Pulmonary Embolism Severity Indices
Like the PESI and sPESI, biomarker data function in the PERT assessment as high-sensitivity, low-specificity predictors of PE-mediated morbidity and mortality. The best-studied serum biomarker is the high-sensitivity troponin-T assay, which was found earlier in the decade to predict 30-day mortality with 87% sensitivity when a cutoff of 14 pg/mL is used.30 More recently, another prospective study confirmed this finding while also suggesting a cutoff of 45 pg/mL for patients older than 75 years to enhance specificity.31 N-terminal pro-brain natriuretic peptide has a similar sensitivity for the prediction of 30-day mortality and adverse events.32
Finally, the echocardiogram is crucial to the assessment of patients with PE and is often performed urgently following PERT activation. Echocardiography is sometimes used to assist in the diagnosis of PE, as up to 20% of PERT activations occur in the context of a high-risk patient for whom a CT diagnosis is unavailable, that is, due to renal insufficiency or contrast allergy.33 In other cases, it is used to confirm that significant symptoms or hemodynamic abnormalities, etc., are attributable to PE and not to some other cause. Its most common application, however, is to further risk-stratify patients with intermediate-risk PE. Two echocardiographic measurements have been validated for this purpose. As with CTA, the ratio of RV-to-LV size is assessed, though it is less sensitive and specific in this setting,34 likely due to the operator-dependence of echo. The clear advantage lies with the assessment of RV systolic function, particularly through measurement of its surrogate marker which is tricuspid annular plane systolic excursion (TAPSE). In a large, prospective study of patients with low- and intermediate-risk PE, abnormal TAPSE values ≤1.6 cm were associated with a hazard ratio of 4.4 for 30-day mortality.35 In another prospective study of a similar patient population, TAPSE measurements ≥2.0 cm had a negative predictive value of 100% for the clinical endpoint and were sufficient to re-stratify patients as low-risk for PE-associated death and rescue thrombolysis. Conversely, a TAPSE ≤1.5 cm was associated with a positive predictive value of 23% and a hazard ratio of 27.9 for this combined endpoint.36 This measurement is easily obtained in most patients and is highly reproducible, accounting for its success in prognostication for PE.
From Assessment to Treatment
The PERT responder's risk assessment determines whether the patient is to be treated with systemic fibrinolytics, catheter-directed fibrinolytics, mechanical thrombectomy, or anticoagulation alone. A detailed account of the interventional management of acute PE would go beyond the scope of the present discussion, but a review of a few key concepts is warranted. For patients with high-risk PE and no risk factors for bleeding, the risk of mortality is high enough to warrant treatment with the maximum-efficacy agent, which is systemic fibrinolysis. However, when contraindications to systemic fibrinolysis are present, or when the treatment fails to restore hemodynamic stability, catheter-directed fibrinolysis provides the ability to administer low-dose fibrinolytics to the site of the thrombus. This treatment protocol requires an infusion of recombinant tissue plasminogen activator over 6 to 12 hours through catheters inserted into the right and/or left pulmonary arteries via the femoral or jugular veins. The total fibrinolytic dose is much lower than that utilized in systemic fibrinolysis, and major bleeding and intracranial hemorrhage occur only rarely (Table 2).6,7
Absolute and Relative Contraindications for Administration of Thrombolytic Therapy (Systemic and Local)*
This approach attenuates bleeding risk sufficiently in most patients, but when absolute contraindications to fibrinolytic therapy are present, as in a patient with a history of intracranial hemorrhage or recent stroke, catheter-directed mechanical thrombectomy (CDMT) may be preferred to fibrinolysis. Thrombectomy may also be preferred in patients for whom hemodynamic instability argues against reliance on slow-infusion catheters for hemodynamic improvement.37 CDMT is typically performed using an aspiration catheter or with a rheolytic device that fragments the thrombus with a saline jet before aspiration. Finally, when the thrombus burden and bleeding risk are both particularly high, surgical embolectomy may be considered.
The optimal management of normotensive patients with pulmonary embolism is not well-defined in the literature, and disagreement exists even among professional societies. Robust studies of catheter-directed fibrinolysis in patients with submassive PE and imaging evidence of RV enlargement have demonstrated decreases in RV size and PA pressure during the index hospitalization in excess of those seen in patients treated with therapeutic anticoagulation alone. Still, data regarding the durability of these benefits are lacking, and no mortality benefit has been reported.6,7 Nevertheless, the most recent guideline statements of the European Society of Cardiology and the PERT Consortium recommend that it be considered in select populations, particularly those with massive or submassive PE and contraindications to systemic fibrinolysis5,38. Our proposed algorithm (Figure 1A) follows theirs in distinguishing between “intermediate-high” and “intermediate-low” risk treatment groups within the broader category of intermediate mortality risk. Accordingly, we recommend that normotensive patients be considered for invasive therapy who have an intermediate mortality risk, positive biomarkers, and imaging evidence of RV dysfunction.
Conclusion
Systemic fibrinolysis is beneficial and potentially lifesaving for patients with massive and submassive pulmonary embolism, but it carries a significant risk of major bleeding and is often contraindicated. Catheter-directed techniques are frequently used in high-risk patients at elevated bleeding risk and in a subgroup of intermediate-risk patients in whom are found both biomarker evidence of myocardial injury and imaging evidence of right heart strain. While the burgeoning presence of PERT programs in the United States promises increased access to a rapid subspecialty response to intermediate- and high-risk PE, risk assessment begins at the first point of contact with these patients and can be potentially lifesaving.
Algorithm for risk assessment and treatment of acute PE*
Notes
This article was externally peer reviewed.
Funding: None.
Conflict of interest: The authors report are no conflicts of interest.
To see this article online, please go to: http://jabfm.org/content/34/2/402.full.
- Received for publication June 23, 2020.
- Revision received September 13, 2020.
- Accepted for publication September 14, 2020.