Abstract
Purpose: Colorectal cancer (CRC) screening is recommended starting at age 45, but there has been little research on strategies to promote screening among patients younger than 50. This study assessed the effect of a multicomponent intervention on screening completion in this age group.
Methods: The intervention consisted of outreach to patients aged 45 to 49 (n = 3,873) via mailed fecal immunochemical test (FIT) (sent to 46%), text (84%), e-mail (53%), and the extension to this age group of an existing standing order protocol allowing primary care nurses and medical assistants to order FIT at primary care clinics in an urban safety-net system. We used segmented linear regression to assess changes in CRC screening completion trends. Patients aged 51 to 55 were included as a comparison group (n = 3,943). Data were extracted from the EHR.
Results: The percentage of patients aged 45 to 49 who were up-to-date with CRC screening (colonoscopy in 10 years or FIT in last year) increased an average of 0.4% (95% CI 0.3, 0.6)) every 30 days before intervention rollout and 2.8% (95% CI 2.5, 3.1) after (slope difference 2.3% [95% CI 2.0, 2.7]). This difference persisted after accounting for small changes in the outcome observed in the comparison group (slope difference 1.7% [95% CI 1.2, 2.2]).
Conclusions: These results suggest that the intervention increased CRC screening completion among patients 45 to 49. Health care systems seeking to improve CRC screening participation among patients aged 45 to 49 should consider implementing similar interventions.
- Colorectal Cancer
- Delivery of Health Care
- Early Detection of Cancer
- Gastroenterology
- Health Services
- Linear Regression
- Middle Aged
- Preventive Medicine
- Primary Health Care
- Quantitative Research
- Safety-Net Providers
- Screening
In response to increasing colorectal cancer (CRC) mortality in younger age groups, in May 2021 the United States Preventive Services Task Force released a statement recommending that average-risk individuals begin colorectal cancer screening at age 45.1 Earlier statements from the task force recommended screening for patients aged 50 to 75. Screening has remained underutilized among all eligible age groups and among younger patients in particular: 58% of patients aged 45 to 75 and 20% of patients aged 45 to 49 were up-to-date with recommended screening in 2021.2
Prior research on interventions to increase screening completion has focused on patients aged 50 or older, finding that the most effective strategies were mailed fecal immunochemical tests (FIT) and patient navigation, sometimes implemented in conjunction with electronic outreach by text or e-mail.3⇓–5 However, there is little information on the effectiveness of any interventions in the 45 to 49 age group, and some interventions have not been well studied in any age group.6 Standing orders allow nurses, medical assistants and other members of the health care team to complete clinical tasks without first obtaining an order from a physician or advanced practice clinician.7 National advisory organizations discuss the use of standing order protocols as a component of comprehensive strategies for increasing completion of CRC screening; however, there have been few studies assessing their impact on this outcome.8,9
In the present study, we used a controlled interrupted time series (ITS) analysis to evaluate the effect of a multicomponent intervention that included outreach with mailed FIT, text messaging and e-mail to a portion of eligible patients aged 45 to 49 and the extension of an existing standing order protocol for FIT to this age group. We hypothesized that the intervention would increase CRC screening completion.
Methods
Study Design and Setting
We used a controlled ITS design to assess the association between intervention rollout and the completion of CRC screening at 11 primary care clinics from the San Francisco Health Network, an urban safety-net health system that provides care to residents of San Francisco County (275,986 primary care visits in fiscal year 2021 to 2022 representing 57,525 unique patients). The network also provides comprehensive specialty care, including gastroenterology (171,444 specialist visits in fiscal year 2021 to 2022). In June 2022, 59% of patients aged 50 to 75 in the network were up-to-date with colorectal cancer screening. This study was approved by the Institutional Review Board at the University of California, San Francisco.
Interventions
A standing order protocol for FIT had been in place for patients 50 to 75 since 2019 within the network and was expanded to patients 45 to 49 on October fourth, 2022. Electronic health record (EHR) reminders became visible to all clinicians and staff flagging patients aged 45 to 49 who were due for CRC screening (no colonoscopy in past 10 years or FIT in last year) and, on the same day, a protocol went into effect that allowed registered nurses, medical assistants and health outreach workers to offer FIT for flagged patients without a physician, nurse practitioner or physician assistant signing an order in advance. Affected staff received training on the expanded FIT protocol before its implementation. Similar standing order protocols and electronic reminders were already in effect for patients within this age group for other preventive care measures.
During this same period from August 2022 to December 2022, the network’s population health unit in collaboration with the gastroenterology division also conducted a mailed FIT and electronic outreach campaign directed toward patients aged 45 to 49. Due to resource limitations, they were only able to mail FIT to 46% of patients aged 45 to 49 due for CRC screening. To address existing disparities, they prioritized patients due for screening on who self-identified as Black in the EHR and mailed them FIT. Using alternating birth month, they selected 50% of the remaining patients to be mailed FIT. The network did not mail FIT to patients who did not have a PCP designated in the EMR or who had a FIT test recently ordered. Packets consisted of a letter with basic information about CRC signed by the patient’s PCP (in English, Spanish or Chinese), the FIT kit, lab requisition, prepaid return envelope, and wordless instructions on completing the test. Patients receiving mailed FIT kits who had a phone number in the EHR were also sent 1 to 2 reminder text messages within 3 weeks of the mailing date. Patients who did not receive mailed FIT kits and had a phone number in the EHR were sent a generic informational text on the day before intervention rollout explaining the new CRC screening guidelines for patients aged 45 to 49 and encouraging recipients to complete screening. In addition, in August before the rollout of the intervention, all patients with an e-mail address in the EHR were emailed an informational flyer encouraging them to complete screening.
Study Period
The study period lasted from October 10, 2021, to May 2, 2023. October 4, 2022, was selected to represent the intervention rollout date. This was the date the standing order protocol went into effect and shortly after the first large group of patients were mailed FIT. There were 20 time points in the study period, spaced 30 days apart (12 before the intervention rollout date).
Data and Sample Selection
All network patients aged 45 to 49 and 51 to 55 were included in the study if they had an in-person or telehealth visit with a physician, nurse practitioner or physician’s assistant at any of the 11 primary care clinics within the prior 2 years. Patient eligibility was assessed at each time point. FIT and colonoscopy results and patient characteristics were extracted from the network EHR. There were no patients with missing outcome data.
Outcome Measures
The primary outcome was the percentage of patients in each age group who were up-to-date with CRC screening. Patients were considered up-to-date with screening at a time point if they had completed a colonoscopy within the past 10 years or a FIT within the last year (in the San Francisco Health Network the number of patients screened by other modalities is very small). They were considered due for screening if they had not done so. The secondary outcome was 30-day FIT completion among patients due for screening, defined as the percentage of patients due for screening who completed a FIT in the 30-day interval after a time point.
Comparison Group
To assess the impact of events other than the rollout of the intervention on the study outcomes, we included patients aged 51 to 55 from the same health system as a comparison group. These patients were not included in the mailed and electronic outreach efforts targeting patients aged 45 to 49. A standing order protocol for CRC screening had been in place for patients aged 50 to 75 since 2019, so there was no change in their exposure to the standing order protocol during the study period. We excluded patients aged 50 to ensure sufficient exposure to the standing order protocol.
Statistical Analysis
We assessed the effect of the interventions on CRC screening using segmented linear regression with ordinary least squares estimation.10 Our regression equation is presented in Appendix 1. For the primary analysis, we fit separate linear trend lines for the percentage of patients up-to-date with screening in the preintervention and post-intervention time periods for ages 45 to 49 and 51 to 55. For each age group we calculated the difference between the preintervention and post-intervention slopes. As the 51 to 55 age group was not exposed to the intervention, we assumed that any observed changes in the outcome in this group would be due to events other than the intervention rollout. To account for the possibility that the outcome in the 45 to 49 age group might also be affected by events other than the intervention rollout, we calculated estimates of the effect of the intervention by subtracting the pre/post-intervention slope difference observed in 51 to 55 age group from the pre/post-intervention slope difference observed in the 45 to 49 age group.11
To assess the effect of the components of the intervention, we repeated the above procedure and performed 3 secondary analyses: 1) excluding patients mailed FIT with percentage of patients up-to-date with screening as the outcome, 2) excluding patients mailed FIT with 30-day FIT completion as the outcome, 3) excluding patients mailed FIT or sent electronic outreach with 30-day FIT completion as the outcome. We did not perform an analysis excluding patients mailed FIT or sent electronic outreach with percentage of patients up-to-date with screening as the outcome because the majority of patients in this analysis would have already been up-to-date with screening in the preintervention period (which is why they did not receive any outreach).
To assess the impact of autocorrelation on our primary analysis, we performed a sensitivity analysis fitting generalized least squared models with autoregressive correlation structures (Appendix 2). We performed subgroup analyses by gender, race/ethnicity, preferred language, and insurance type (Appendix 3). All analyses were completed using R statistical software (4.2.3).
Results
We analyzed 11,343 patients meeting inclusion criteria at a minimum of 1 time point. On the date of intervention rollout (October 4, 2022), there were 7,816 (69%) unique patients (3,873 [50%] aged 45 to 49 and 3,943 [50%] aged 51 to 55) meeting inclusion criteria (Figure 1). Among these 672 (17%) patients aged 45 to 49 and 1,934 (49%) patients aged 51 to 55 were already up-to-date with screening and 3,201 patients aged 45 to 49 and 2009 patients aged 51 to 55 were due for screening. Of all patients aged 45 to 49 who were due for screening, 1,489 (47%) were mailed FIT, 2,787 (87%) were sent text messages, 1,884 (53%) were sent e-mail and 392 (12%) did not receive any outreach (Table 1 shows patients receiving each combination of outreach components). Patients were racially and ethnically diverse and approximately half reported a primary language other than English (Table 2). Almost all patients had government-sponsored insurance.
Participant flow. Abbreviation: FIT, fecal immunochemical test.
Patient Characteristics at Time of Intervention Rollout, by Age Group
Number of Patients Aged 45–49 Due for Screening on Date of Intervention Rollout That Received Each Component of the Intervention
Percentage of Patients Up-to-Date with Screening
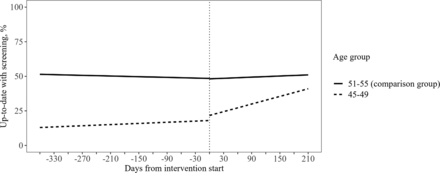
As shown in Figure 2 and Table 3, in the preintervention period the percentage of patients aged 45 to 49 who were up-to-date with CRC screening was increasing by an average of 0.4% (95% CI 0.3, 0.6) every 30 days. After intervention rollout, the percentage of patients up-to-date with screening began to increase more rapidly with an average change of 2.8% (95% CI 2.5, 3.1) every 30 days (slope difference 2.3% [95% CI 2.0, 2.7]). The percentage of patients aged 51 to 55 up-to-date with screening also began to increase more quickly after the introduction of the intervention for patients aged 45 to 49, but the change was smaller in the older group (slope difference 0.7% [95% CI 0.4, 0.9]). The pre/post-intervention difference in trend observed in the 45 to 49 age group persisted after subtracting the pre/post-intervention difference observed in the 51 to 55 age group (slope difference 1.7% [95% CI 1.2, 2.2]). After excluding patients exposed to mailed FIT outreach, we found that the rollout of the standing order protocol continued to be associated with faster growth in the percentage of patients up-to-date with screening compared with the preintervention period (slope difference 1.0% [95% CI 0.7, 1.3]; slope difference after accounting for comparison group 0.3% [95% CI 0.0, 0.7]).
Percentage of patients up-to-date with colorectal cancer screening before and after intervention rollout. Includes all analyzed patients. Dashed lines represent pre- and post-intervention regression lines for the percentage of patients aged 45 to 49 up-to-date with screening. Solid lines represent pre- and post-intervention regression lines for the percentage of patients aged 51 to 55 up-to-date with screening. The vertical dotted line represents the date of intervention rollout.
Effect of Interventions on the Percentage of Patients up-to-Date with Screening
30-day FIT Completion
As shown in Table 4 and Figure 3, among patients aged 45 to 49 who were not mailed FIT kits (intervention consisted of standing order protocol with or without electronic outreach), the intervention rollout was associated with an immediate increase in the 30-day FIT completion (intercept difference 3.3% [95% CI 2.3, 4.3]) but a small decrease in the 30-day FIT completion trend (slope difference −0.2% [95% CI −0.4, 0.0]). Among patients aged 51 to 55, after the screening intervention for patients aged 45 to 49 was introduced, there was a small immediate increase in 30-day FIT completion (intercept difference 0.7% [95% CI −0.3, 1.7]) and a small increase in the 30-day FIT completion trend (0.1% [95% CI −0.1, 0.4]). Pre/post-intervention differences observed in the 45 to 49 age group persisted after subtracting pre/post-intervention differences observed in the 51 to 55 age group (intercept difference 2.6% [95% CI 1.2, 4.0]; slope difference −0.3% [95% CI −0.7, 0.0]). In our analysis excluding patients who received mailed or electronic outreach (intervention consisted of standing order protocol only), intervention rollout was still associated with an immediate increase in the 30-day FIT completion rate (intercept difference 2.5% [95% CI 1.3, 3.7]; intercept difference after accounting for comparison group 1.8% [95% CI 0.1, 3.5] and a small decrease in the 30-day FIT completion trend (slope difference −0.2% [95% CI −0.5, 0.0]; slope difference after accounting for comparison group −0.4% [95% CI −0.8, 0.0]).
Thirty-day Fecal Immunochemical Test (FIT) completion before and after intervention rollout. Excludes patients who received mailed FIT. Dashed lines represent pre- and post-intervention regression lines for 30-day FIT completion for patients aged 45 to 49. Solid lines represent pre- and post-intervention regression lines for 30-day FIT completion for patients aged 51 to 55. The vertical dotted line represents the date of intervention rollout.
Effect of Standing Order Protocol and Electronic Outreach on 30-Day FIT Completion Rate, Excluding Patients Who Were Mailed Fecal Immunochemical Test (FIT)
Sensitivity and Subgroup Analyses
In a sensitivity analysis accounting for autocorrelation (Appendix 2), 95% confidence intervals for all estimates of the effect of the intervention on screening completion were wider, but did not cross zero, except for the adjusted pre/post-intervention difference in the trend in the percentage up-to-date with screening among patients aged 45 to 49 who did not receive mailed FIT (adjusted slope difference 0.6% [95% CI −0.4, 1.5]). In subgroup analyses (Appendix 3) we observed that, in comparison to other groups, effect estimates tended to be higher for female patients, for Latino/a and White patients, for patients with a preferred language of Spanish and for patients with Medicare.
Discussion
In a controlled interrupted time-series analysis, a multicomponent intervention including outreach with mailed FIT, text messaging and e-mail as well as a standing order protocol gradually increased the percentage of patients up-to-date with CRC screening among patients aged 45 to 49 in an urban safety-net health system. In secondary analyses excluding patients who received mailed FIT and electronic outreach, we found that the standing order protocol still had an immediate positive effect on 30-day FIT completion.
Mailed FIT, electronic outreach and standing order protocols are commonly used to increase screening completion among adults aged 50 and older. These findings suggest that these interventions will be successful in patients aged 45 to 49 as well. The multicomponent intervention that we studied led to a 1.7% (95% CI 1.2, 2.2) absolute increase in the average 30-day change in the percentage of patients up-to-date with screening after accounting for changes in the outcome observed in the comparison group. Extrapolating this suggests that the interventions increased the absolute percentage of patients aged 45 to 49 who were up-to-date with screening by 12% over the duration of the 7-month post-intervention period (from a baseline of 17% on the date of intervention rollout). Although it is not possible to directly compare effect sizes due to differences in study design, our results are consistent with the findings of prior studies demonstrating the effectiveness of multicomponent outreach interventions to increase CRC screening completion among adults 50 and older.3 Our results are also consistent with 1 small study of mailed FIT among patients 45 to 49 that showed that after the intervention was implemented the percentage of patients up-to-date with screening increased by 16.6% (95% CI 10.9, 22.3), from 26.7% at baseline to 43.3% at 6 months.6
The results of our secondary analyses suggest that the standing order protocol had a positive impact on screening completion, even without mailed FIT or electronic outreach. In all these analyses, we observed an increase in screening participation from preintervention to the post-intervention period. We observed a slight decrease in the slope of the 30-day completion rate after intervention rollout. Immediately after intervention rollout there were many unscreened patients. With time, 30-day FIT completion may have decreased slightly as the number of unscreened patients decreased and the composition of those remaining shifted to patients who were less responsive to the standing order protocol.
We observed small changes in several outcomes in our comparison group of patients 51 to 55. These changes could be due to events that occurred at the same time as intervention rollout, affecting screening completion in both the intervention group and the comparison group. For instance, increases in primary care visits in the wake of the COVID-19 pandemic could have led to increasing trends in screening participation in the post-intervention period in both age groups. Our effect estimates incorporating changes in the outcomes in the comparison group account for this possibility and indicate that intervention was still effective even after accounting for changes in outcomes observed in the comparison group. Another possibility is that observed changes in the comparison group may represent unintended effects of the interventions. For instance, it is possible that intervention rollout may have led clinic staff to pay increased attention to CRC screening for all patients leading to the small increase in the post-intervention trend in the percentage of patients up-to-date with screening observed among patients aged 51 to 55. If this were the case, effect estimates that do not incorporate changes observed in the comparison group may be more accurate than those that do.
Limitations
We note several limitations. First, although the comparison group of patients aged 51 to 55 shared many relevant characteristics with the intervention groups of patients aged 45 to 49 (same health care system, similar demographic characteristics), there were also differences. Notably, older patients had higher baseline screening rates and a longer history of exposure to CRC screening outreach. It is possible to imagine events occurring at the time intervention rollout that, because of these differences, would have lead to different sized effects on screening participation among each group. If this occurred, our effect estimates that account for changes in the comparison group could be biased. We were unable to include a comparison group of patients aged 45 to 49 because 1) although some patients were not exposed to all elements of the intervention, exposed and unexposed patients were likely different in important ways. For instance, patients who did not have an e-mail address in the EHR or who did not have a clinical encounter that would have exposed them to the standing order protocol were likely less connected to primary care than patients who were exposed to these intervention components. 2) We did not have access to data for patients aged 45 to 49 from a comparable outside system (a safety net system in the same geographic region).
Second, the post-intervention period of our study was only 7 months, so we were unable to assess the long-term effect of the standing order protocol. Third, EHR reminders, the standing order protocol and electronic outreach were implemented for all patients aged 45 to 49 at the same time, so it was not, possible to separately estimate the effect of each of these intervention components. Fourth, we only had access to FIT and colonoscopy results from the San Francisco Health Network EHR. If patients completed FIT or colonoscopy in another health care system, we did not have access to those results. Fifth, our secondary analyses excluding patients mailed FIT may have underestimated the effect of the intervention. Because the sample size was smaller in these analyses, a higher proportion of patients completed screening in the preintervention period which reduced the observed change in screening participation from the pre- to post-intervention periods. Finally, our study was conducted within 1 safety-net health care system. Results may not be generalizable to other settings with different characteristics.
Implications
CRC mortality is increasing among younger patients. Recommendations to start screening at age 45 may help to reduce this trend, but only if individuals participate in screening. Our findings suggest that mailed FIT, electronic outreach and standing order protocols could be effective interventions for health care systems seeking to improve CRC screening participation among patients aged 45 to 49. Randomized controlled trials including patients aged 45 to 49 are needed to confirm these findings.
Although national advisory organizations endorse the use of standing order protocols to increase CRC screening participation, there have been few prior studies in any age group assessing their effectiveness. The results of a subgroup analysis of patients who were exposed only to the standing order protocol provides evidence to support these recommendations. Future studies of the impact of standing order protocols on CRC screening completion should include all age-eligible patients, focus on a variety of clinical settings and assess cost-effectiveness.
Conclusions
Mailed FIT, electronic outreach and standing order protocols are commonly employed strategies to increase CRC screening completion, but their effectiveness has not previously been studied among patients aged 45 to 49. Our findings suggests that these strategies have the potential to be effective in this age group.
Appendix 1 – Regression Model
For each analysis, we constructed a regression model with the following formula:
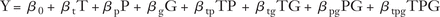
Where:
Y = the outcome of interest (percentage of patients up-to-date with screening or the FIT completion rate)
T = time point (with T = 0 corresponding to intervention rollout)
P = a dummy variable indicating if an observation corresponded to the preintervention period (P = 0) or the post-intervention period (P = 1)
G = a dummy variable indicating if an observation corresponded to intervention group of patients aged 45 to 49 (G = 0) or the comparison group of patients aged 51 to 55 (G = 1)
We used the following linear combinations of regression coefficients to estimate differences between the preintervention trendlines and post-intervention trendlines
Age 45 to 49 pre/post-intercept difference = βp
Age 45 to 49 pre/postslope difference = βtp
Age 51 to 55 pre/post-intercept difference = βp + βpg
Age 51 to 55 pre/postslope difference = βtp + βtpg
Aged 45 to 49 pre/post-intercept difference accounting for comparison group = βpg
Age 45 to 49 pre/post slope difference accounting for comparison group = βtpg
Appendix
Appendix 2 – Sensitivity Analyses for Autocorrelation
Analysis
We performed a sensitivity analysis to assess the impact of autocorrelation on our estimates. If autocorrelation is present in a time series, ordinary least squares regression may lead to confidence intervals that are too small.12 To assess for autocorrelation, we used the residuals of the ordinary least square models described in the main text and the forecast package for R to iteratively fit autoregressive integrated moving average (ARIMA) models with different correlation structures and selected the model with the lowest Bayesian information criteria score.13 If this procedure identified autocorrelation, we used the identified correlation structure and the reduced maximum likelihood method without Satterthwaite correction to fit a generalized least squares model to re-estimate the effects of the interventions.12 This procedure was performed to the unadjusted pre/post-intervention difference in all groups as well as adjusted pre/post-intervention differences in the 45 to 49 age group. As in the main results, adjusted differences were estimated by subtracting the pre/post-intervention difference observed in the 51 to 55 age group from the pre/post-intervention difference observed in the 45 to 49 age group.
Results
We found evidence of first order autocorrelation in both models used to estimate the effect of the interventions on the percentage of patients up-to-date with screening. Using generalized least square models, the 95% confidence intervals for all estimates of the effect of the intervention on screening completion among patients 45-49 were wider, but did not cross zero, except for the adjusted estimate of the effect of the standing order protocol and electronic outreach on screening completion among patients aged 45-49 who did not receive mailed FIT. In this group the lower limit of the 95% confidence interval for the unadjusted pre/post-intervention difference remained greater than zero (1.1% [95% CI 0.5, 1.8]), but the 95% confidence interval for adjusted pre/post-intervention difference crossed zero (0.6% [95% CI −0.4, 1.5]). We did not find evidence of autocorrelation in the model with the 30-day FIT completion rate as the outcome.
Effect of Interventions on the Percentage-up-to-Date with Screening After Accounting for Autocorrelation, With and Without Adjustment for Changes in the Comparison Group
Appendix 3 – Subgroup Analyses
Effect of Interventions on the Percentage of Patients up-to-Date with Screening, Subgroup Analysis by Gender
Effect of Interventions on the Percentage of Patients up-to-Date with Screening, Subgroup Analysis by Race/Ethnicity
Effect of Interventions on the Percentage of Patients up-to-Date with Screening, Subgroup Analysis by Race/Ethnicity
Effect of Interventions on the Percentage of Patients up-to-Date with Screening, Subgroup Analysis by Insurance Type
Effect of Standing Order Protocol and Electronic Outreach on 30-Day FIT Completion Rate excluding Patients Who Were Mailed Fecal immunochemical Test (FIT), Subgroup Analysis by Gender
Effect of Standing Order Protocol and Electronic Outreach on 30-Day Fecal immunochemical Test (FIT) Completion Rate excluding Patients Who Were Mailed Fecal immunochemical Test (FIT), Subgroup Analysis by Race/Ethnicity
Effect of Standing Order Protocol and Electronic Outreach on 30-Day Fecal immunochemical Test (FIT) Completion Rate excluding Patients Who Were Mailed Fecal immunochemical Test (FIT), Subgroup Analysis by Preferred Language
Effect of Standing Order Protocol and Electronic Outreach on 30-Day Fecal immunochemical Test (FIT) Completion Rate excluding Patients Who Were Mailed FIT, Subgroup Analysis by Insurance Type
Notes
This article was externally peer reviewed.
Funding: Funding for the outreach interventions was provided by the San Francisco General Hospital Foundation. Funding for the outreach interventions and for data extraction was provided by the San Francisco Cancer Initiative (SFCAN). SPM was supported by Health Resources and Services Administration (HRSA) grant (T32 HP 19025).
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/37/4/660.full.
- Received for publication November 1, 2023.
- Revision received February 24, 2024.
- Accepted for publication March 4, 2024.