Abstract
Background: Individuals with hearing loss (HL) are at higher risk for hospitalizations, and may be for readmissions, compared with their hearing peers. The objective of this prospective study was to confirm retrospective studies suggesting that HL increases hospital readmissions, and, if confirmed, possible causes for it.
Methods: A prospective cohort study of English-speaking patients >55 years old admitted to general medical and surgical floors at 2 large hospital systems in southeastern Michigan over a 2-year period was conducted. All patients underwent bedside audiometric testing. HL presence and severity were categorized using World Health Organization pure tone assessment parameters. Readmission rates, Charlson comorbidity index, socio-demographic and medical variables were obtained from Epic EMR databases.
Outcomes: There were 1247 hospitalized patients enrolled. Of these, 76.8% had documented HL of which 50.5% (630) was mild HL and 26.3% (328) moderate or worse HL. Patients with any HL were older and more likely to be non-Hispanic, white, male, and had less education, lower health literacy, more comorbidities, and more difficulty communicating with their doctor. Readmission rates at 30 and 90-days were similar between HL and hearing groups, after adjusting for HL severity, Charlston index, and numerous potential confounders.
Conclusion: Patients with HL do not seem to have higher rates of hospital readmissions. We did find high frequency of HL in hospitalized patients along with significant communication difficulties that patients had with their clinicians. These findings have implications for measures to improve patient-physician communication, potentially improving long-term health outcomes.
- Disability
- Hearing Loss
- Hospitalization
- Michigan
- Patient Readmission
- Prospective Studies
- Retrospective Studies
Introduction
Hearing loss (HL), America’s second most prevalent disability, affects 17% of Americans, most of whom have mild or moderate loss.1⇓–3 HL is common in older people4 (>50% for those at least 70 years of age), most of whom are untreated or unidentified as having HL5⇓–7 and most of whom do not have hearing aids, even when those are recommended.8,9 Despite HL’s high prevalence, its negative impact on health outcomes is generally unappreciated. Most physicians are unaware that HL (at all levels of HL) is associated with poorer cognitive function,10,11 poorer physical health,12,13 functional decline,14 impaired social interaction,15,16 social support systems dependence17 and higher hospital admission rates.18
People with HL have substantial communication difficulties. Moreover, due to stigma or embarrassment, they often pretend to understand conversation or instructions, when they do not.17,19 This can adversely impact health outcomes if hospitalized patients do not understand physicians’ instructions, follow-up plans, and cautions. Physicians do not screen for HL – either in office or hospital settings - despite the availability of reliable screening tests.7,20,21 They are also uncomfortable addressing it.21,22 Thus, because most patients with mild/moderate HL refrain from mentioning it,19 they are usually not identified when hospitalized.23 This is a concern because hospitalized patients with HL have higher morbidity.24⇓⇓–27 Of note, providing amplification devices to these patients has been shown to improve communication with nurses and physicians.28
Studies suggest that people with mild (16% higher) and moderate (21% higher) HL have a higher risk of hospitalization than their hearing peers.18 Moreover, they are more expensive; on a per capita basis, individuals with HL have $22,000 in additional health care costs added over 10 years versus those with normal hearing, even after adjusting for a variety of sociodemographic and health factors.29 Patients with HL who use hearing aids have 9% lower hospitalization rates than those who do not,30 supporting the importance of identifying and treating HL to reduce hospitalization rates and improve communication and health outcomes. Other studies have also documented the health benefits of using hearing devices; Tiase showed that using these reduces the risk of falls during hospitalizations31 in patients with HL.
Nationally, ∼13.9% of hospital admissions are readmissions, leading to substantial costs, morbidity and mortality.32 Many of these are preventable.33 Certain diagnoses are known to increase readmission risk including diabetes, COPD, chronic kidney disease, and heart failure.34,35 Retrospective studies suggests untreated HL or self-reported communication difficulties increases the risk of hospital readmission beyond the specific medical condition for which patients are admitted. A review of the 2010 to 2013 Medicare Current Beneficiary Survey (MEPS) found a 32% greater risk of hospital readmission in patients ≥65 years with HL who had “trouble communicating” with medical personnel.36 Reed found individuals with untreated HL had a 44% higher 30-day readmission rate.29 However, these studies had no audiologic HL verification in the hospital, no determination if hospitalized individuals with HL used a hearing device there, some had lower rates of identified HL than expected, and all were retrospective raising questions of study accuracy and missing important covariates (eg, health literacy) that may contribute to increased readmissions. Hsu et al note there is insufficient evidence to support that HL is independently associated with increased readmissions and studies are needed to better understand this.37
Here we report on a prospective study examining the association of HL with hospital readmission. We measured hearing levels directly and obtained a variety of other data to evaluate whether the presence of HL is associated with hospital readmission.
Methods
This study was conducted in 2 major hospital systems in southeastern Michigan: University of Michigan Health System and Beaumont Health. Institutional Review Board approvals were obtained at both institutions. The study occurred over a one and a half year time period (September 2019 to April 2021), interrupted twice for infection control reasons due to high Covid censuses.
Patients >55 years old, who were primary admission patients (ie, no previous admissions within 30 days) on medical and surgical floors at both participating hospitals, were identified electronically every weekday morning. The research assistants approached eligible patients, and if they spoke English and consented, enrolled them in the study; patients not speaking English were excluded. If patients were unavailable, for example, getting a test, too ill to talk, had family with them, meeting with their medical team, or were isolated due to infection precautions, the research assistants returned as feasible. Our 2 hospitals’ Average Length of Stay (ALOS) of ∼3.5 days provided multiple opportunities to reach patients. Approximately 13% of eligible patients approached declined participation; the remainder who did not participate were due to 1 or more of the above reasons.
Our research assistants asked each patient: “We are doing a study on hospital readmissions. It takes about 10 to 15 minutes, and involves you answering 12 short questions and having your hearing tested.” If the patient agreed, they were first asked up to 12 questions (Figure 1). Patients denying HL were asked 10 questions (they were not asked #3 and #4) and those stating they had HL were asked 12 questions. Each question involved 1 to 3 word answers. The questionnaire included an assessment of each patient’s health literacy and ability to communicate with doctors. Patients were asked how much trouble they have communicating with doctors or other medical professionals and responded on a 3-point scale of no trouble, a little trouble, or a lot of trouble. Health literacy was assessed using the question regarding confidence in filling out medical forms with options on a 5-point Likert scale ranging from not at all comfortable to extremely comfortable.
Questions asked of subjects.
Then, to determine HL presence, all patients underwent Shoebox Audiometry in their hospital room.
Shoebox audiometry data were used to characterize patients’ HL using the World Health Organization (WHO) Criteria.38 Pure Tone Average (PTA) was calculated by averaging the hearing sensitivity (dB) at 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz thresholds. The better of the 2 ears was used to categorize HL as none (PTA<=25), mild (25<PTA<=40), moderate (40<PTA<=60), severe (60<PTA<=80), and profound (PTA > 80). The average total visit took 10 to 15 minutes. All survey question answers and audiograms were recorded on iPads and uploaded to our database.
The following additional data were obtained through each hospital’s data office via a search of their electronic medical records: a) readmission within 30 or 90-days after hospital discharge; b) Charlson comorbidity index score (identifies complexity of the admissions and readmissions); c) discharge diagnoses; d) demographic information (age, gender, ethnicity); e) insurance type.
Descriptive statistics were used to summarize the sociodemographic and relevant hospitalization variables, stratified by having no HL, mild HL, or moderate HL or higher using WHO definitions. Kaplan Meier survival curves were run for 30-day and 90-day readmission rates and results were compared with presence/absence of any HL as well as across WHO defined HL levels using a log-rank test. Kaplan-Meier and log-rank tests were also used to compare survival functions between individuals with HL who did vs did not have HL devices for both 30 and 90-day readmissions.
Cox Proportional Hazard models were used to look at the impact of HL on the hazard functions for 30 and 90-day readmission adjusting for comorbidities based on Charlson index (0, 1, or 2 or more comorbidities), age (in years), gender, race, years of education, health literacy (continuous 5-point scale), whether they had trouble understanding their doctor (3-point response; treated categorically) and hospital system. Impact of HL was assessed as both a binary indicator (any evidence of HL) as well as the 5-point WHO criteria severity scale. Analogous models were run for the subset of patients with HL, focusing on the covariate of whether or not they used HL devices. All analysis was performed using Stata 17.39
Results
There were 1247 patients enrolled (39% of those eligible), of whom 289 (23.2%) had no level of HL based on their pure tone average (PTA), 630 (50.5%) had mild HL and the remaining 328 (26.3%) had moderate or higher HL. Patients with any evidence of HL were on average older and disproportionately non-Hispanic, white and male. They also tended to have lower average education and a higher number of comorbidities. In addition, individuals with HL reported having a harder time communicating with their doctor and were less confident in filling out medical forms (lower health literacy). Readmission rates at both 30 and 90-days were similar between HL groups (Table 1), including after adjusting for Charlston index scores.
Patient Characteristics
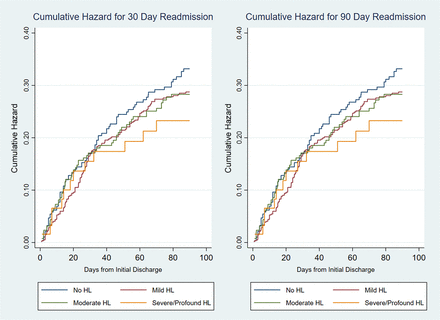
We looked at a 3-category variable (known HL, no HL, unknown HL) using subject’s response to question 3 above: “yes” to question #3 (known HL), “no” to #3 but positive audiogram (unknown HL), and “no” to #3 and normal audiogram (no HL). There were no associations found with readmission. Survival curves (Figure 2), using a log-rank test, found no difference between the HL severity groups for the risk of 30-day (P = .98) or 90-day readmissions (P = .61). Similarly, analysis using dichotomous HL (any vs none) found no evidence of a difference in readmission risk for 30 (log-rank P = .83) or 90-day (log-rank P = .23) time periods.
Cumulative hazard readmission. Abbreviation: HL, Hearing Loss.
Cox Proportional Hazard models run for both 30 and 90-day readmissions found no association of HL when treated as a binary indicator (p-value = 0.51 and 0.14, respectively – see Table 2) or when severity of HL was taken into account (30-day; P = .83) and 90-day; P = .32)
Cox Proportional Hazard Model for Readmission at 30 and 90 Days, by any Hearing Loss (HL)
Among patients with a documented HL based on their PTA score on Shoebox, 198 (20.7%) reported having a hearing device, 471 (49.2%) reported not having a device but suspected they had HL, and the remaining 289 (30.2%) said they did not have HL and were considered to not have a device. No significant association existed between 30 or 90-day readmission and having a HL device. (Table 3) Although not significant, the direction of the point estimate suggested a trend to reduced readmission rate if a HL device was used.
Cox Proportional Hazards on Readmission at 30 and 90 Days, among Those with HL Who Use an Hearing Loss (HL) Device
Since people with HL are known to have lower health literacy, lower income and other social determinants of health, we assessed these variables in our population. Patients with HL were more likely to have lower literacy (question #7 - Difficulty completing medical forms; P < .001), food insecurity (question #10 - Within the past 12 months, the food you bought did not last and you did not have money to get more; P = .03), and trouble communicating with their physicians (question #6 - How much trouble do you have communicating with your doctor or other medical personnel; P < .001). None of these, however, were associated with readmission rates.
Discussion
In this prospective study, we found no difference in readmission risk between patients with and without HL – whether looking at 30 or 90-day time frames. We are not aware of a previous published prospective study that conducted an objective hearing assessment of an entire group of hospitalized patients to evaluate the impact of HL on readmission risk. Our findings of no differences in hospital readmission rate contrast those published from retrospective studies cited earlier. Similarly, there was no impact of HL with survival rates.
Of interest, 77% of patients in our study had a HL based on their PTA values. Genther et al also reported high rates of HL in hospitalized patients; they found a 59% HL rate in their cohort of hospitalized patients, with higher rates of admission with increasing HL severity.18 In that study, HL was determined before patients were hospitalized (ie, as an outpatient). One possibility for our slightly higher rate of HL in hospitalized patients than Genther is that our audiologic screening may have been impacted by ambient noise in the hospital rooms. Although Shoebox audiometry is known to be accurate in assessing the degree of HL,40,41 and patients wore ear-muffs to minimize the impact of ambient noise, we did not measure the ambient noise. It is possible that ambient noise made it harder for some patients to hear the tones and thus were misclassified as having HL. Another potential explanation for our higher HL rates is that patients were more fatigued, due to being in the hospital, which would make it harder to concentrate on the audiogram tones.
Our findings, consistent with many others, suggests that HL increases the risk for initial hospitalization, though we did not directly measure that. If true, reasons for this may be complicated. There are unique drivers that could explain higher risk of initial hospitalizations among those with HL. Having HL is associated with numerous medical and social determinants of health, as outlined in our introduction. We also found that patients with more severe HL had higher Charlson comorbidity scores. It is possible that other specific medical conditions – more than the direct impact of HL itself – causes the higher rate of hospital admissions. However, we also found that food insecurity and lower health literacy were more prevalent in patients with HL; others have shown lower health literacy in these patients.42 Previous reports have shown that people with HL report higher unmet medical needs, delays in getting medical needs met, less access to care, and having unfilled prescriptions compared with those without HL.43 Any of these social determinant risks may contribute to the higher hospitalization risk for people with HL.
Another possible cause is the communication difficulty these patients have. We found that those with HL had more trouble communicating with their physicians, from 39% for those with mild HL to 57% for those with moderate or worse HL (P < .001). Patients provided numerous comments about difficulties understanding physicians and nurses. Here are 2 typical comments:
When the doctor types on the computer and faces away from me while talking (usually when answering their questions), it is difficult to hear them.
Accents, as well as difficulty hearing the doctor makes it just so hard to understand what the doctor means.
Of interest, when these patients were asked if they shared this with their health care team, most expressed their embarrassment to us or deemed it as a nuisance. Further studies are needed to understand the implications of the communication barrier of patients with HL on longer-term health outcomes, including hospitalization rates. Since most patients with HL are embarrassed about it and do not tell their physicians19 and most physicians are uncomfortable dealing with HL, that makes efforts to address this more complex as well as more needed.19,44
We noted a suggestive trend toward lower readmissions in patients with HL who used a hearing device (hearing aids, cochlear implant), consistent with findings by others,27,28 though this was not statistically significant. Use of hearing devices in the United States, due to cost, is associated with socioeconomic status. Thus, a measure of SES will need to be included in future studies to truly understand if such devices reduce readmissions. If the use of hearing devices is confirmed in future studies to reduce readmissions, this would buttress the need for more support for widespread screening for HL in primary care and push for coverage of HL devices by insurers, to increase the number of patients who use them. We do note that the USPSTF rated screening for HL an “I” due to lack of information that population-based screening for HL clearly leads to use of HL devices with improved outcomes.45 Implementing screening will need to deal with the fact that most patients with HL will not admit their loss unless prompted by physicians,19,44 most physicians do not screen for it,21,22 and patients with HL often do not use hearing assistive devices.22,46 Future studies are needed to determine the best ways to generate more use of hearing assistive devices by those who would benefit, then see if doing that improves health outcomes.
There are limitations to this study. We were only able to enroll 39% of all eligible patients. Although only 13% of patients declined to participate when asked, numerous other patients were out of the room for tests, too sick to participate, had visitors/medical team present, did not speak English or were not approachable because of access restrictions due to COVID-19 or other illness issues. This introduces a potential selection bias in that the 61% of patients who did not participate in our study may be different, that is, may be sicker, had more or less HL, were not English speaking, or had higher readmission rates versus who did participate. Sicker patients, such as those in the ICU, were not included and thus our findings do not apply to them. Patients with HL who did not speak English may have different readmission rates too.
Another limitation is that audiologic screening may have been slightly less accurate due to ambient noise in hospital rooms, as discussed earlier. In addition, although in our approaching patients we specifically did not state that we were looking at the impact of HL on readmission (see methods for specifics of our “pitch”), we did inform patients that we would test their hearing. It is possible that patients who suspected they had HL may have been more likely to participate (the results were private in that they were not shared with hospital staff or family, and thus there was no stigma involved). Moreover, it is likely that some readmissions occurred at other hospitals and were unknown to us. Whether this occurred at the same rate for those with and without HL is unknown. The major likelihood of this is for patients who live long distances from the 2 hospitals (both major referral centers) and we did not obtain information regarding where patients lived. In addition, those patients surveyed during the COVID-19 pandemic portion of the study may have different (fewer) readmission rates compared with those who were surveyed prepandemic due to the increased effort to keep people out of the hospital. Finally, we did not look at multiple readmission rates but rather focused on initial readmissions during the 30 or 90 day time periods.
In conclusion, the presence of HL, documented by direct audiologic screening in hospital rooms, was associated with numerous problems such as poor communication with physicians, lower health literacy and high prevalence rates in hospitalized patients. However, HL was not associated with increased readmissions in this prospective study.
Notes
This article was externally peer reviewed.
This is the Ahead of Print version of the article.
Funding: This study was funded by Blue Cross Blue Shield Foundation of Michigan grant number 00278.II
Conflict of interest: The authors of this manuscript have no conflicts of interest, financial or otherwise.
To see this article online, please go to: http://jabfm.org/content/00/00/000.full.
- Received for publication December 5, 2022.
- Revision received February 14, 2023.
- Accepted for publication February 17, 2023.