Abstract
Introduction: We analyzed data from a prospective cohort of older primary care patients to determine whether the presence of peripheral neuropathy (PN) was associated with premature mortality and to investigate potential mechanisms.
Methods: PN was defined as the presence of 1 or more bilateral lower extremity sensory deficits detectable by physical examination. Mortality was determined from key contacts and Internet sources. Statistical models were used to evaluate the association between PN and mortality.
Results: Bilateral lower extremity neurological deficits were common, reaching 54% in those 85 and older. PN was strongly associated with earlier mortality. Mean survival time for those with PN was 10.8 years, compared with 13.9 years for subjects without PN. PN was also indirectly associated through impaired balance.
Conclusions: In this relatively healthy cohort of older primary care patients, PN detectable by physical examination was extremely common and strongly associated with earlier mortality. One possible mechanism involves loss of balance, though our data were insufficient to determine whether poor balance led to injurious falls or to less-specific declines in health. These findings may warrant further studies to determine the causes of age-associated PN and potential impact of early detection and balance improvement and other fall prevention strategies.
- Aged
- Geriatrics
- Life Expectancy
- Peripheral Nervous System Diseases
- Peripheral Neuropathies
- Primary Health Care
- Prognosis
Introduction
The prevalence of peripheral neuropathy (PN) increases with advancing age. Using data from the 1999-2004 National Health and Nutrition Survey and the 2016-2017 Atherosclerosis Risk in Communities Study, Hicks and colleagues1 estimated that 10.4% of middle-aged adults and between 26.8% and 39.2% of older adults had PN based on abnormal monofilament test results. Although longstanding diabetes mellitus is often a contributor, there are many other causes, and in a large proportion of people, the primary cause is never identified.
The Oklahoma Longitudinal Assessment of Health Outcomes of Mature Adults (OKLAHOMA Studies) was a prospective cohort study involving patients 64 to 102 years of age recruited from primary care practices in central Oklahoma in 1999. A goal of the study was to determine the prevalence, predictors, and consequences of PN. Large amounts of subjective information and objective measures of cognitive and peripheral neurological function in the lower extremities as well as gait and balance were collected. Deaths have continued to be tracked since enrollment.
Baseline prevalence of bilateral lower extremity neurological deficits was 26% in 64 to 74-year-olds, 36% in 75 to 84-year-olds, and 54% in those 85 and older.2 Follow-up analyses in 2007 found that individuals with bilateral peripheral neurological deficits were more likely to fall, had higher rates of hospitalization, and had greater reductions in quality of life and self-rated health during the study period and higher mortality rates over an average of 4.8 years.3 However, only 11.5% of participants had died. The purpose of the current analyses was to reassess the associations now that 80% of the participants have died. If confirmed, we proposed to further examine potential explanations, and in particular, pathways from PN through balance to declines in health and falls as potential mechanism for mortality, using structural equation modeling (SEM.).
Methods
Study Data
We described the OKLAHOMA Studies methodology in greater detail in a prior publication.2 Between January 1, 1999, and December 31, 2000, 23 family physician members of the Oklahoma Physicians Resource/Research Network created from their billing records lists of patients 65 years of age and older seen by them within the prior 18 months. Patients were excluded if they had switched physicians, had died, were in nursing homes, or were believed by their PCP to be too confused to sign consent. Eligible patients received a letter from their physician inviting them to participate. Two weeks later, the project coordinator contacted interested patients via telephone. The study was reviewed, approved, and monitored by the Institutional Review Board of the University of Oklahoma Health Sciences Center, and all subjects consented to participate, first by telephone and then by signing a written consent form. Approval was obtained from the Institutional Review Board.
Those who agreed to participate were asked to complete a questionnaire sent to them before their enrollment visit. It included questions about demographics, health habits, symptoms, and medical conditions. Two registered nurses then enrolled participants in the offices of their family physicians. They reviewed the study protocol, obtained informed consent, and checked the questionnaire for completeness. Each year on the anniversary of their initial enrollment, participants were invited to re-enroll. Those who agreed completed a follow-up questionnaire and were re-examined by 1 of the nurses. No new participants were added after the second year.
The baseline physical examination included height, weight, blood pressure and pulse in 3 positions, and examinations of fine touch in the feet, position sense in the great toe, vibration at the medial malleoli, and deep tendon reflexes at the ankles. Examinations were performed by 2 research nurses trained by a neurologist and validated on test patients. Gait was assessed using a timed 50-foot walk, and balance was scored using the Tinetti Balance Scale.4 PN was defined as the presence of bilateral sensory deficits.2
Deaths were determined at the end of the initial study period using information provided by participants’ designated contacts, their primary care physicians, and from the Social Security Death Index (http://ssdi.rootsweb.ancestry.com). Subsequently, deaths have been tracked using publicly available online databases (Ancestry.com, FindAGrave.com, familysearch.org, Google.com, and whitepages.com) through December 31, 2021.5
Descriptive statistics were calculated for baseline variables. Student’s t-test, Chi-square, and nonparametric Kruskal-Wallis tests were used to analyze bivariate associations between baseline variables and PN and between baseline variables and death. Due to the number of comparisons a P value of less than 0.01 was considered to be statistically significant. Logistic regression was used to identify variables associated with death. Life-table analyses and plots were used to compare mortality curves. Cox proportional hazards models6 were then created by considering promising independent predictors. A stepwise linear regression was used to identify possible predictors of survival time. All analyses were performed using SAS Studio v94.
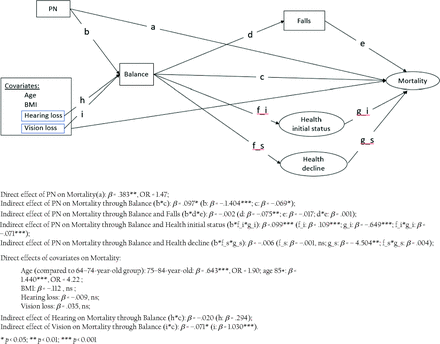
In evaluating both the direct and indirect impact of PN on mortality, a generalized structural equation model that included latent growth curve and discrete-time survival models (Figure 1) was constructed7⇓–9 using Mplus version 8.8 software. Two indirect impacts of PN on mortality (eg, path b -> d ->e) were assessed along with its direct impact (ie, path a). Both indirect pathways involved balance and its downstream influence on mortality through falls or through self-rated health status. Age, body-mass index (BMI), vision loss, and hearing loss were included covariates in the model. For the pathway through health status, growth modeling allowed examination of both initial health status and health decline (ie, path b -> f_i -> g_i and path b -> f_s ->g_s).
A path diagram of the hypothesized mediation model. Abbreviation: BMI, body mass index.
Results
Study Population
Seven hundred ninety-nine individuals completed the initial questionnaire and physical assessments. Reasons for annual attrition from the study population included dying, change in physical status, such as becoming incapacitated or other changes, admission to a nursing facility, or declining to participate further in the study. PN status was available for 796 participants.
PN (Table 1) was strongly associated with older age, height, greater BMI (over 30 kg/m2 vs less than 30), history of military service, diabetes mellitus, rheumatoid arthritis, poorer self-rated health, slower timed walk, and worse balance scores, and a history of hereditary neuropathy. It was not associated with race, alcohol consumption, or cigarette use. Balance was strongly associated with PN, visual impairment, and hearing impairment, but PN was not associated with visual or hearing impairment.
Variables Potentially Associated with Peripheral Neuropathy (PN)
By the end of 2021, 641 (80%) participants had died. The dataset, with respect to mortality, includes up to a maximum of 22 calendar years and 10,334 person-years of follow-up. Mean and median survival times were 12.9 years and 13.0 years, respectively. As in the 2007 analysis, the dichotomous variable, death, was independently associated with older age, having lower self-rated health at baseline, slower timed gait, and lower Tinetti balance score. PN was also significantly associated with the risk of death (P = .0003).
Mean survival time for those with PN was 10.8 (SD, 6.5) years, compared with 13.9 (SD, 6.5) years for subjects without PN (P < .0001). Life-table analysis showed significantly different survival curves for participants with PN versus those without it (Wilcoxon test: P < .0001). The unadjusted Kaplan-Meier curves are shown in Figure 2.
Kaplan-Meier survival for subjects with and without peripheral neuropathy (PN).
Cox proportional hazard models were constructed to assess the contribution of baseline variables to survival time. Initial models showed that decreased survival was associated with older age, male gender, being unmarried, having lower self-rated health, cigarette use, poorer balance scores at enrollment, and having PN at the time of enrollment. The final model (Table 2), eliminating nonsignificant variables, identified an association between mortality and the same 7 prior significant variables. However, significant interaction terms of self-rated health and balance scores with survival time were identified and incorporated into the model.
Cox Proportional Hazards Model for Survival Time for Subjects with Peripheral Neuropathy versus Those without Peripheral Neuropathy
SEM results suggest that, controlling for the impact of age, BMI, vision loss, and hearing loss, PN had significant direct and indirect effects on mortality. For PN patients, the odds of dying increased by 47% (a: β = 0.383***, OR = 1.47), while controlling for indirect influences involving balance. PN predicted poor balance, which later predicted a higher mortality rate (b*c: β = 0.097**). Poor balance was also associated with poor health, which led to a higher mortality rate (ie, b*f_i*g_i: β = 0.099***). Falls were associated with poor balance (d: β = –0.075**), but having fallen during follow-up did not (independently) predict mortality (e: β = –0.017). Health decline was not associated with balance (f_s: β = –0.001) but did predict mortality (g_s: β = – 4.504**). For descriptive information on the variables used in the model, refer to Mold, et al.3 Although not depicted in Figure 1, we did also test indirect pathways that bypassed balance. These indirect pathways were not significant, in large part, due to small direct influences of PN on falls and on health status. In addition, we tested the impact of vision and hearing loss on mortality through balance. Vision loss was associated with reduced balance, which led to reduced life expectancy (i*c: β = –0.071*). Compared with our youngest age group (64 to 74 years old), the odds of dying increased by 90% (β = 0.643***, OR = 1.90) for those age 75 to 84 years and increased by 322% (β = 1.440***, OR = 4.22) for those 85 or older.
Discussion
In this relatively healthy, well-educated, cognitively intact cohort of primary care patients aged 64 to 102, PN was associated with earlier mortality, after controlling for baseline age, marital status, gender, race, military service, current alcohol intake, smoking history, and self-rated health. The association between PN and premature mortality was independent of common causes of neuropathy, considered separately or as a combined variable.
The diagnosis of bilateral PN was made by physical examination. While more sensitive tests (eg, nerve conduction studies) are available, they are not routinely used for this purpose in primary care settings. We did not conduct extensive diagnostic evaluations to determine the etiologies of participants’ PN, but, because the most common causes were not associated with mortality, it is unlikely that the less common causes would have contributed substantially to our findings or conclusions.
The prospective cohort study design precludes establishment of causation. However, a great deal of prior research has shown that PN impairs balance and increases risk of falls in older people.10⇓⇓⇓⇓–15 Individuals with impaired balance tend to reduce physical activity, which may secondarily reduce their intake of key nutrients and reduce sleep quality.16,17 They are also less likely to participate in activities outside of their homes, leading to social isolation.18 In an earlier analysis of OKLAHOMA Studies data, we found that PN was associated with more emergency department visits and hospitalizations.3 If the consequences of PN are lethal, it would behoove clinicians to regularly screen patients by physical examination and encourage behaviors that reduce risk of falling, such as Tai Chi and balance and lower extremity strengthening exercises.19,20
It is, of course, possible that idiopathic age-associated PN is simply a marker of biological aging because the prevalence increases with age. A majority of participants over 85 years of age had bilateral peripheral neurological deficits. Pathophysiological studies have identified age-related changes in the expression of myelin proteins and reduced ability to regenerate damaged myelin.17,21 Similar changes have been noted in other aging tissues. Special sensory losses such as vision or hearing were not associated with PN in this study. Cognitive issues may be an important covariable, but inclusion in our analysis is beyond the scope of this study.
Because of similarities in the type and distribution of the involved nerves, some have proposed that a common cause of age-related PN is undiagnosed diabetes.22,23 A related possibility is that damage to the peripheral nerves results from progressive glycosylation of proteins due to long-term glucose exposure in individuals with normal glucose levels, a process that has been proposed to contribute to biological aging.24,25
Several secondary findings from these analyses deserve further study. The association of prior military service and PN is intriguing. Because it held even after controlling for income and education, it raises the question of whether some cases of PN were the result of exposure to toxins encountered during military service. However, 98% of those with a history of military service were male, and there was a significant interaction between gender and PN in the proportional hazards model. The association of being currently unmarried with increased mortality, which is consistent with the findings from other studies, also deserves further investigation to determine whether risk mitigation is possible.26
Strengths of our study are its prospective cohort design, a nearly complete dataset, an unselected primary care population, the high proportion of deaths, and our ability to control for a number of relevant covariates and confounders. We believe that we captured all or very nearly all deaths, but the possibility exists that some deaths were missed. We are confident in the neurologic examination data based on the rigorous training, calibration, and examination procedures, and there is evidence that neurologic examination is both sensitive and specific for electrophysiologically confirmed deficits.27
The study was limited in several ways. Participants were not rigorously evaluated to determine the causes of their neuropathy. We did not have access to medical records or causes of death for the population. Participants who fell and had significant injuries may well have dropped out of the study, making it impossible for us to connect those particular dots in our SEM analysis.
Although we captured many of the known predictors of survival time, including a number of medical conditions, we did not have information on the duration or severity of those conditions. Our population size may have been too small to capture rare or uncommon characteristics or underlying conditions.
Conclusions
In a population of relatively healthy older subjects, having PN is associated with increased mortality and reduced life expectancy. Based on our data and available literature, the most significant, potentially remediable reason seems to be the adverse consequences of the neuropathy rather than its causes. Older patients should be routinely and carefully assessed for the physical signs of PN and, if present, they should be counseled to begin balance improvement exercises like Tai Chi.
Notes
This article was externally peer reviewed.
This is the Ahead of Print version of the article.
Funding: Presbyterian Health Foundation and the University of Oklahoma Department of Family and Preventive Medicine.
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/00/0/000.full.
- Received for publication September 9, 2022.
- Revision received January 18, 2023.
- Accepted for publication January 20, 2023.