Abstract
Background: There are multiple classes of pharmacologic agents approved for treatment of osteoporosis, but their costs vary widely, and systematic data on their efficacy compared with the traditional standard, bisphosphonates, for reducing fractures in postmenopausal women are lacking. The objective was to perform a systematic review and meta-analysis assessing the efficacy of denosumab compared with bisphosphonates.
Methods: Researchers selected randomized controlled trials (RCTs) comparing denosumab to bisphosphonates that included information on clinical and/or osteoporotic fracture events over the follow-up period. Each clinical outcome was meta-analyzed using a fixed-effects analysis, with clinical and osteoporotic fractures as the outcomes of interest. A meta-regression was performed using change in bone mineral density (BMD) as the moderator variable.
Results: Seven RCTs were included. Denosumab was not associated with a reduction in clinical or osteoporotic fractures compared with bisphosphonates. There was no association between the change in BMD with denosumab and bisphosphonates and denosumab’s effect on both osteoporotic and clinical fractures.
Discussion: Existing data do not support the use of the more expensive denosumab as a first-line agent over bisphosphonates for reduction of fractures in postmenopausal women with osteoporosis. One limitation in this study was each RCT was not individually powered for fracture incidences.
Introduction
Osteoporosis is a common condition affecting nearly one quarter of postmenopausal women in the United States. It is associated with significant morbidity and mortality from fragility fractures.1 There are multiple classes of pharmacologic agents designed to reduce fracture risk in patients with osteoporosis, but their costs vary widely, and systematic data on the comparative efficacy of these agents for fracture reduction are lacking. Bisphosphonates cost approximately $960/year and historically have been considered the standard of care, but over the last decade, denosumab has entered the market. The mechanism of action by which bisphosphonates protect bone is multifactorial. By attaching to a hydroxyapatite binding site on bony surfaces, they impair the ability of bone-resorbing osteoclasts to attach. In addition, bisphosphonates reduce osteoclast recruitment and promote osteoclast apoptosis.2 Denosumab (brand name Prolia) is a monoclonal antibody that inhibits Receptor activator of nuclear factor kappa-B (RANK) ligand and costs approximately $3000/year.3 RANK ligand is an osteoclast differentiating factor. When RANK ligand binds to its receptor, activation of bone-resorbing osteoclasts occurs.4 The American College of Physicians (2017) and the American Academy of Clinical Endocrinologists (2016) consider both bisphosphonates and denosumab first-line agents.5 If value is defined as outcome over cost, the efficacy of denosumab in reducing fractures must be 3 times greater than that of bisphosphonates to justify its use.6
In the United States, insurance companies have established a stepwise hierarchy of pharmacologic intervention within some medical subspecialties. Coverage options incentivize providers to order the least expensive treatment options before moving on to more expensive alternatives. For example, with regards to cardiovascular disease, a first-line lipid altering agent such as a statin must be trialed before insurers will cover the more costly PSK-9 inhibitor evolocumab (brand name Repatha). However, there is no hierarchy in place to drive prescribing patterns with regards to osteoporosis therapies in this country.
The aim of this systematic review, meta-analysis, and meta-regression is to appraise the quality of evidence and aggregate data from randomized controlled trials (RCTs) to test the hypothesis that denosumab is superior to bisphosphonates for reducing fractures in postmenopausal women with osteoporosis. Furthermore, to test the hypothesis that greater increases in bone mineral density (BMD) will be associated with less fracture, a meta-regression will be performed using the percent change in BMD between denosumab and bisphosphonates as the moderator variable.7,8 Conclusions from this study will aide health care providers and policy makers in selecting antifracture agents for clinical use in postmenopausal women with osteoporosis.
Methods
Data Sources
We followed the PRISMA statement for reporting systematic reviews and meta-analyses.9 A systematic literature search was conducted in Medline and PubMed for English-language RCTs of denosumab published from January 1, 2000 to September 2, 2020 (see search terms in the Appendix). We also searched the references of all articles retrieved. We identified all RCTs of denosumab versus bisphosphonates for postmenopausal women with osteoporosis that included information on clinical and/or osteoporotic fracture events over the follow-up period and information on BMD.
Study Selection
Two authors independently performed the following steps to screen studies identified in the database search and extract data. Any disagreements were resolved by consensus. First, all titles were reviewed to exclude studies that were observational, used computational methods to model outcomes, or included subjects from a population outside of this study’s interest (eg, patients with osteoporosis following chemotherapy). Next, the full text of all remaining studies was reviewed, using the same exclusion criteria mentioned above as well as excluding studies that did not report event rates for either clinical and/or osteoporotic fractures.
Data Extraction
Two authors independently reviewed all studies meeting inclusion criteria and performed standardized data extraction of the following study characteristics: main inclusion criteria, design (eg, route of administration, interval and dosing in intervention and comparator arms), primary endpoint(s), length of follow-up, and patient outcomes (clinical fractures and osteoporotic fractures). An osteoporotic fracture is synonymous with a fragility fracture, or 1 occurring due to diminished bone density (ie, vertebral compression fractures, radius fractures of wrist, hip fractures). They may be associated with little to no trauma and are referred to as “low-energy fractures” in the orthopedic literature. These fractures may be subclinical (especially in the case of vertebral compression fractures) and sometimes are only detected on routine imaging/surveillance. A clinical fracture is synonymous with a traumatic fracture and includes any bodily fracture sustained from high-energy impact/trauma.10 Mean percent change in BMD was also extracted from each trial. Regardless of the primary outcome of the trial, fracture was the primary outcome of this meta-analysis. If a trial did not report mean percent change in BMD, this was then calculated by using absolute BMD values at baseline and at the end of the trial.
Study Quality
Two authors independently assessed study quality using Cochrane Collaboration’s tool for assessing risk of bias in randomized trials.11 The bias assessment was performed at the level of outcomes for this systematic review and meta-analysis, not the primary endpoint of the individual studies. Any disagreements were resolved by consensus. Information on study quality and risk of bias was synthesized in the qualitative synthesis, which was integrated with the quantitative results to facilitate conclusions and determine confidence levels.
Data Synthesis
The Mantel-Haenszel method was used to conduct the primary analysis. Each clinical outcome was organized into a 2 × 2 table and meta-analyzed on the log relative scale using a fixed-effects model. The principal summary measure was the odds ratio (OR) of experiencing a clinical or osteoporotic fracture for patients receiving denosumab compared with bisphosphonates. Examination of heterogeneity was performed using Q statistics and I2. Prespecified sensitivity analyses were performed for each clinical outcome by excluding each study individually from the analysis and examining its effects on the summary effect and heterogeneity. A fixed-effects meta-regression was performed using the percent change in BMD at both the hip and spine between denosumab-treated patients and bisphosphonate-treated patients, and the end of the study was used as the moderator to determine if this continuous variable was associated with the treatment effect of denosumab on clinical and osteoporotic fracture. The following statistical tests were used in the meta-regression: τ2, which estimates the true variance among RCTs; I2; and R2 index, which is the proportion of variability between study variance explained by the moderator. In addition, regression coefficients were reported that describe how denosumab’s treatment effect on clinical and osteoporotic fracture changes with a unit change in the moderator variable. Meta-regression linear graphs are displayed by plotting the moderator variable on the x-axis and the treatment effect of denosumab on the y-axis (the log of the OR). When assessing the log of the OR, a value of zero is an OR of 1, a negative value corresponds to an OR < 1, and a positive value corresponds to an OR > 1. Examination of publication bias was performed visually using funnel plots.
Statistical Analysis
Statistical analyses were performed with the computer program Review Manager (RevMan) version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The P values were 2-sided, with an α value of 0.05 considered significant. The meta-regression was performed using Comprehensive Meta-Analysis version 3 (Englewood, NJ: Biostat, 2013).
Results
Qualitative Synthesis
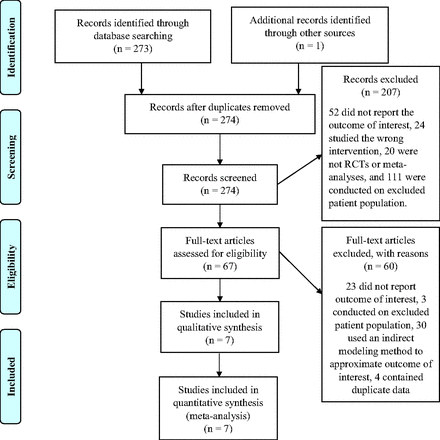
We screened 274 records and 67 full-text articles, from which 7 RCTs that randomized 4635 patients were included: 2457 randomized to denosumab and 2178 to bisphosphonate12⇓⇓⇓⇓⇓–18 (Appendix Figure 1). All trials involved postmenopausal women with osteoporosis defined by T-scores; the most common criteria was a T-score between -4 and -2 at the lumbar spine, femoral neck, or total hip. The mean age of participants was not provided in the published manuscripts of any trials; however, inclusion for 4 trials required women ≥55 years of age. Six trials followed participants for 12 months and 1 trial for 48 months (Table 1).
Description of Trials
The primary endpoint was defined by BMD in 6 trials; 4 assessed for percent change in BMD over the follow-up period and 2 used absolute BMD values. The primary endpoint of 1 trial was defined by medication adherence. Four trials required use of bisphosphonates for some period of time before randomization; 2 of the 4 trials required poor compliance with bisphosphonates before randomization.
In 6 trials denosumab was given 60 mg subcutaneously once every 6 months. One trial was a phase 2 dose-finding trial in which denosumab was given over a range of doses from 3 mg to 210 mg subcutaneously either every 3 months or every 6 months. In 4 trials, the comparator was alendronate given orally at 70 mg once weekly; in 2 trials ibandronate 150 mg oral tablets taken monthly or risedronate 75 mg oral tablets taken every 2 weeks were used, and in another trial zoledronic acid 5 mg given intravenously once yearly was used.
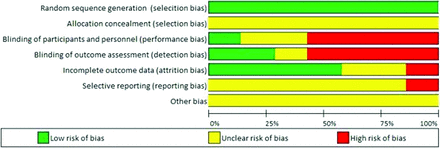
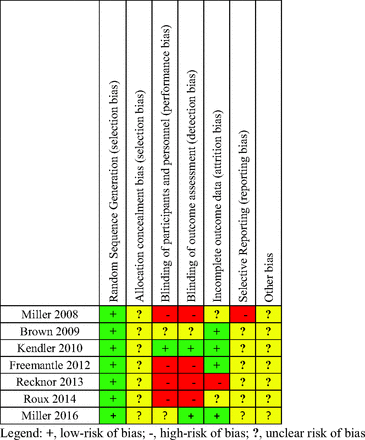
There was variation in trial quality based on the Cochrane Collaboration’s tool for bias assessment, with most trials having moderate to high risk of bias (Appendix Figure 2). Four trials were nonblinded, making them highly susceptible to performance and ascertainment bias. Allocation concealment (the process of concealing randomization status until the moment of assignment) was not explicitly described in the published manuscripts of any of the trials, therefore all trials were of unclear risk of bias for allocation concealment (Appendix Figure 2). Not concealing allocation assignment increases the risk of selection bias. In addition, all trials were industry funded.
Industry funding was noted as a possible risk of “other bias” because industry-sponsored trials are nearly 4 times more likely to report positive results than nonindustry-sponsored studies.19
Quantitative Synthesis
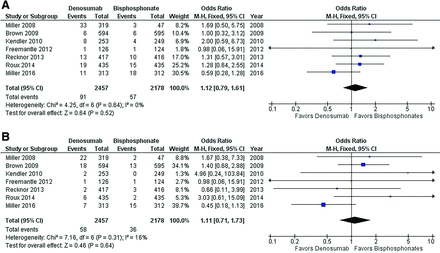
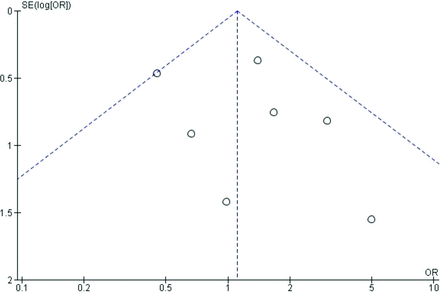
Denosumab was not associated with a reduction in clinical fractures compared with bisphosphonates (3.7% vs 2.6%; OR 1.12; 95% CI, 0.79-1.61). There was no significant heterogeneity between trials assessed by χ2 (4.25, P = .64 or I2 0%) (Figure 1a). The overall effect estimate is not sensitive to the exclusion of any individual trial. There was no evidence of publication bias based on visual inspection of the funnel plot. Exclusion of the dose finding trial by Miller et al yields similar results for clinical fractures when comparing patients treated with denosumab to bisphosphonates (2.7% vs 2.5%; OR 1.07; 95% CI, 0.74-1.56) with no evidence of heterogeneity (χ2 3.76, P = .58; I2 0%).12
Forest plot of denosumab versus bisphosphonates for clinical and osteoporotic fractures. A: Clinical fracture data. B: Osteoporotic fracture data. The forest plot represents the odds ratio for fracture events among participants randomized to denosumab versus bisphosphonates.
Denosumab was also not associated with a reduction in osteoporotic fractures compared with bisphosphonates (2.4% vs 1.7%; OR 1.11; 95% CI, 0.71-1.73). There was evidence of mild heterogeneity with a χ2 7.16 (P = .31) and I2 16%. (Figure 1b) The overall effect estimate is not sensitive to the exclusion of any individual trial. There was no evidence of publication bias based on visual inspection of the funnel plot. The heterogeneity estimate is sensitive to exclusion of the trial by Miller et al with I2 being reduced to 0% from 16%.18 With exclusion of this trial, the effect estimate increased to 1.54 from 1.11 in favor of bisphosphonates but did not reach statistical significance.17 Exclusion of the dose finding trial by Miller et al yields similar results for osteoporotic fractures (1.7% vs 1.6%; OR 1.06; 95% CI, 0.66-1.69) with a slight increase in heterogeneity (χ2 6.8, P = .24; I2 27%).11
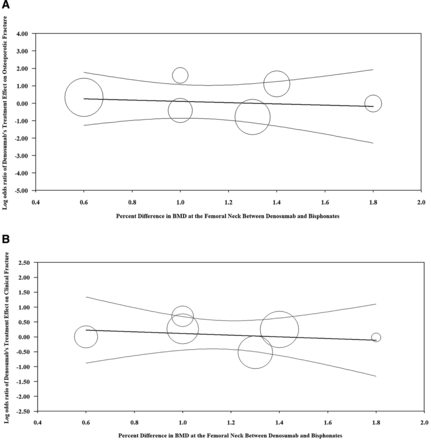
When accounting for percent change in total BMD in the meta-regression model, there was no linear relationship between change in total hip BMD and denosumab’s effect on osteoporotic fracture (τ2=0.0915, I2=16.0%, R2=0.00, regression coefficient = 0.43 [95% CI, -0.71 to 0.99]) (Figure 2a). There was no association between change in total hip BMD and denosumab’s effect on clinical fracture (τ2=0.00, I2=0.0%, R2=0.00, regression coefficient = 0.16 [95% CI, -0.45 to 0.76]) (Figure 2b). In addition, there was no relationship between change in vertebral BMD and denosumab’s effect on osteoporotic fracture (τ2=0.092, I2=16.0%, R2=0.0, regression coefficient = 0.18 [95% CI, -0.42 to 0.78]) or clinical fracture (τ2=0.00, I2= 0.0%, R2=0.00, regression coefficient = 0.16 [95% CI, -0.45 to 0.76]).
A: Regression of log odds ratio of denosumab’s treatment effect on osteoporotic fracture on percent difference in BMD between denosumab and bisphosphonates. B: Regression of log odds ratio of denosumab’s treatment effect on clinical facture on percent difference in total hip BMD between the denosumab and bisphosphonate arms. Fixed effects meta-regression. The x-axis is the percent change in total hip bone mineral density (BMD) between denosumab and bisphosphonates. The y-axis represents the treatment effect of denosumab (log odds ratio) on osteoporotic fracture (A) and clinical fracture (B). Each circle represents an included randomized controlled trial, and the size of the circle represents the weight of the study in the regression model. The dark center line is the regression line and the lighter outer lines are the 95% CI.
Discussion
In summary, when considering both the qualitative and quantitative results, the authors have moderate confidence that denosumab does not reduce clinical or osteoporotic fractures compared with bisphosphonates in postmenopausal women with osteoporosis (Table 2). We cannot exclude the possibility that denosumab could reduce both fracture types over a longer follow-up period, as most trials followed patients for only 12 months. Furthermore, in those randomized to denosumab, greater increases in BMD in both the hip and spine were observed when compared with those randomized to bisphosphonates. Interestingly, this increase in BMD was not associated with denosumab’s effect on fracture reduction. This finding is contrary to that of another meta-analysis composed of 38 RCTs comparing the efficacy of 19 different osteoporosis medications to placebo (not to other drugs), which did report an association between change in mean percent difference in BMD and incidence of fracture using a linear model. This study found strong linear associations between change BMD and vertebral and hip fractures but not other nonvertebral fractures. The findings of Bouxsein et al prompted a “status update” in 2018 by the Food and Drug Administration, which began to consider change in BMD a surrogate marker for fracture reduction.20 Recently, the FDA approved a biomarker qualification plan to use BMD as a surrogate for fractures in trials of new osteoporosis drugs, which is problematic.
Evidence Summary Table for Denosumab versus Bisphosphonates
The results of our study differ from the prior meta-regression. One reason for why our study did not find an association between mean change in BMD and fracture reduction is the small difference in BMD between bisphosphonates and denosumab. However, when comparing 1 drug to placebo, as analyzed by Bouxsein et al, the change in BMD was greater and associated with fracture reduction in a linear manner.20
The findings from this systematic review and meta-analysis advance our understanding of the clinical efficacy of these drugs compared with the traditional standard of care, bisphosphonates. Prior reviews have focused on surrogate endpoints related to changes in BMD and bone turnover markers. Our results are consistent with those reported in meta-analyses by Wu et al and Beaudoin et al, who found no benefit with administration of denosumab compared with bisphosphonates in reduction of fracture risk.21,22 Similarly, a meta-analysis by Lin et al reported no reduction in fracture risk after 1 year of treatment with denosumab compared with the same duration of therapy with alendronate.23 This is not the first analysis to find that a therapy is capable of increasing BMD without reducing fracture. In the Women’s Health Initiative calcium and vitamin D randomized trial, there was also an increase in BMD at the hip with no reduction in fractures among 36,282 postmenopausal women randomized to calcium and vitamin D supplementation versus placebo.24
Providers and policy makers should be cautious of therapeutics that are promoted based on improving surrogate endpoints only when making treatment decisions. While surrogate endpoints may be useful targets for identifying promising therapeutics in the development stage, treatment decisions should be based on evidence of improvements in hard, patient-centered outcomes. The ultimate purpose for use of antifracture agents in postmenopausal women with osteoporosis is to reduce fractures, not simply to increase BMD or bone turnover markers. Historically, there are many examples of medical practices that have been instituted based on surrogate endpoints that have gone on to be reversed after finding they do not improve hard endpoints and in some cases lead to worse results.25
Based on the price tag of individual agents and the results of this meta-analysis, we can infer denosumab is not cost-effective and is lower value compared with bisphosphonates in reducing fractures in postmenopausal women with osteoporosis and thus should be reconsidered as a first-line agent. The authors acknowledge, however, other variables factor into assessing a medication’s utility. For example, dosed once every 6 months, denosumab has a higher compliance rate compared with many of the bisphosphonates (eg, oral alendronate, which requires once-weekly dosing).26 On the other hand, the use of denosumab requires patients to get serum calcium levels checked within 10 days of each injection. Biannual blood draws certainly have adherence requirements and associated costs as well. It is also important to consider the potential side effects of bisphosphonates, which include joint pain, esophageal irritation, body aches, fever, osteonecrosis of the jaw, and atypical femur fractures.27⇓⇓⇓⇓⇓–33
The overall treatment duration (and the cost associated with it) should also be considered. At present, the recommended treatment duration of bisphosphonates is 5 years, and discontinuation of a bisphosphonate does not lead to immediate bone loss.12,34 To the contrary, initiation of denosumab requires indefinite treatment, as discontinuation or delay in injection results in rapid bone loss. An increase in vertebral fractures has been seen as early as 7 months after the prior dose.35 For this reason, it is recommended patients remain on denosumab lifelong or be started on a bisphosphonate within 2 to 3 months of discontinuing denosumab.36 Even in cases where women have a history of intolerance to a certain bisphosphonate, there are many alternatives with different routes of administration and dosing schedules that could be considered before denosumab, including alendronate, zoledronic acid, ibandronate, or risedronate.
Limitations to this meta-analysis include the low number of trials and limited follow-up with varying formulations and dosages of both denosumab and bisphosphonates. In addition, most of the included trials had a primary endpoint of BMD and were not designed to detect differences in fracture, therefore these results should be interpreted with caution. The meta-analysis allowed us to analyze 242 total fractures (148 clinical and 94 osteoporotic fractures), but the possibility remains that our analysis is underpowered. Another limitation is the variability in medication dose and administration frequency (Table 1). For this analysis all doses and medication frequencies were pooled. Given that most trials used alendronate and the remaining trials each used a unique bisphosphonate, subgroup analyses were unable to be performed. In addition, subgroup analyses or regressions were unable to be performed based on follow-up time as the majority of trials followed patients for 12 months, 1 trial followed patients for 48 months, and another trial followed patients for 24 months. The percent change in BMD was regressed; however, lack of significant heterogeneity among the treatment effects noted for the included studies argues against a single covariate exerting any significant interaction effect. One exception may be trial duration, particularly in the case of denosumab, as only two trials followed patients for longer than 12 months. Future trials intended to address hard endpoints should give consideration to this factor. This analysis is also limited by lack of patient-level data to perform prespecified analyses based on potentially important modifying traits. However, the same caveat to this limitation applies, which is the lack of significant heterogeneity, arguing against this possibility.
In conclusion, this is the most comprehensive systematic review and meta-analysis we are aware of that assesses the efficacy of denosumab compared with the traditional standard of care, bisphosphonates, for reducing clinical and osteoporotic fractures in postmenopausal women with osteoporosis. Based on the results of this review, there is limited evidence to support denosumab as a first-line alternative to bisphosphonates. Despite denosumab increasing BMD at both the hip and spine in all trials, denosumab did not reduce fracture compared with bisphosphonates. These results should be used to guide providers and policy makers in selecting antifracture agents in postmenopausal women with osteoporosis.
Appendix. Denosumab Search Terms and Appendix Figures 1-5
Appendix Table of Contents
1. Denosumab search terms in PubMed
2. Appendix Figure 1. PRISMA diagram of trials comparing denosumab to bisphosphonates.
3. Appendix Figure 2. Risk of bias graph for trials comparing denosumab and bisphosphonates.
4. Appendix Figure 3. Risk of bias summary for trials comparing denosumab and bisphosphonates.
5. Appendix Figure 4. Funnel plot of denosumab versus bisphosphonates trials for clinical fractures.
6. Appendix Figure 5. Funnel plot of denosumab versus bisphosphonates trials for osteoporotic fractures.
Denosumab Search Terms in PubMed
(“Alendronate”[Mesh] OR “Zoledronic Acid”[Mesh] OR “Ibandronic Acid”[Mesh] OR “Risedronic Acid”[Mesh] OR Alendronate OR Zoledronic Acid OR Ibandronate OR Risedronate OR bisphosphonates) AND (“Denosumab”[Mesh] OR Denosumab) AND (Meta-Analysis[ptyp] OR Randomized Controlled Trial[ptyp] OR systematic reviews[ptyp] OR Systematic Reviews OR Meta-Analysis OR Randomized Controlled Trial)
PRISMA diagram of trials comparing denosumab to bisphosphonates. RCTs, randomized controlled trials.
Risk of bias graph for trials comparing denosumab and bisphosphonates.
Risk of bias summary for trials comparing denosumab and bisphosphonates.
Funnel plot of denosumab versus bisphosphonates trials for clinical fractures.
fractures.
Notes
This article was externally peer reviewed.
This is the Ahead of Print version of the article.
Funding: None.
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/00/0/000.full.
- Received for publication March 9, 2022.
- Revision received September 25, 2022.
- Accepted for publication September 27, 2022.