Abstract
Purpose: Clinical trials generally have not assessed efficacy of long-term opioid therapy (LTOT) beyond 6 months because of methodological barriers and ethical concerns. We aimed to measure the effectiveness of LTOT for up to 12 months.
Methods: We conducted a retrospective cohort study among adults with chronic low back pain (CLBP) from April 2016 through August 2022. Participants reporting LTOT (>90 days) were matched to opioid nonusers with propensity scores. Primary outcomes involved low back pain intensity, back-related disability, and pain impact measured with a numerical rating scale, the Roland-Morris Disability Questionnaire, and the Patient-Reported Outcomes Measurement Information System, respectively. Secondary outcomes involved minimally important changes in primary outcomes.
Results: The mean age of 402 matched participants was 55.4 years (S.D., 11.9 years), and 297 (73.9%) were female. There were 119 (59.2%) LTOT users who took opioids continuously for 12 months. The mean daily morphine milligram equivalent dosage at baseline was 36.7 (95% CI, 32.8 to 40.7). There were no differences between LTOT and control groups in mean pain intensity (6.06, 95% CI, 5.80-6.32 vs 5.92, 95% CI, 5.68-6.17), back-related disability (15.32, 95% CI, 14.55-16.09 vs 14.81, 95% CI, 13.99-15.62), or pain impact (32.51, 95% CI, 31.33-33.70 vs 31.22, 95% CI, 30.00 to 32.43). Correspondingly, LTOT users did not report greater likelihood of minimally important changes in any outcome.
Conclusions: Using LTOT for up to 12 months is not more effective in improving CLBP outcomes than treatment without opioids. Clinicians should consider tapering opioid dosage among LTOT users in accordance with clinical practice guidelines.
- Chronic Pain
- Low Back Pain
- Opioids
- Patient Reported Outcome Measures
- Registries
- Retrospective Cohort Studies
Introduction
Low back pain affects over 500 million persons worldwide and remains the leading cause of disability.1,2 Major guidelines have shaped treatment of chronic pain in the United States, including for low back pain. The Centers for Disease Control and Prevention (CDC) Guideline for Prescribing Opioids for Pain in 2016 recommended that both nonpharmacological and nonopioid treatments be initiated before using opioids for chronic pain.3 The CDC’s updated guideline in 2022 expanded their prior recommendations, including guidance on opioid selection, dosages, duration of prescribing, and follow-up.4 This guideline also provided updated conversion factors for determining morphine milligram equivalent (MME) dosages and noted that the lowest starting daily dosage for opioid-naïve patients is often 20 to 30 MMEs and that many patients do not experience benefit in pain or function by increasing opioid dosages to ≥50 MMEs.4 The American College of Physicians Clinical Practice Guideline issued in 2017 that addressed noninvasive treatments specifically for chronic low back pain (CLBP) similarly recommended nonpharmacological treatments and nonsteroidal anti-inflammatory drugs before considering opioids.5
A systematic review of long-term opioid therapy (LTOT) for chronic pain was commissioned by the National Institutes of Health (NIH) and reported in 2015.6 Therein, LTOT was defined as >90 days of use in adults with chronic pain, and it found insufficient evidence to determine the effectiveness of LTOT for improving pain and function. More recent clinical reviews and commentaries have continued to question the benefits of LTOT for patients with CLBP.7,8 Ideally, participants in clinical trials involving chronic pain management should be followed for up to 12 months.9 However, before 2018, no randomized controlled trial had assessed LTOT beyond 6 months.10 Although the SPACE trial subsequently found no benefits in treating musculoskeletal conditions with LTOT over 12 months, its generalizability was limited by recruiting predominantly male participants from Veterans Affairs clinics.11
Given the need for evidence on LTOT effectiveness in contrast with its known risks,4 and paucity of long-term data from randomized controlled trials,10 we conducted a registry-based study to assess its effectiveness among patients with CLBP over 12 months.
Methods
Study Design and Participants
This retrospective cohort study included participants selected from the Pain Registry for Epidemiological, Clinical, and Interventional Studies and Innovation (PRECISION) from April 2016 through August 2022. Registry participants were screened and recruited from the 48 contiguous states and District of Columbia, primarily through social media advertising (eg, Facebook) that directed respondents to a Web landing page that included a link to the screening questionnaire (Appendix Figure 1). Both screenees and participants who were eventually enrolled in the registry were asked to use its digital research platform for electronic capture of self-reported data. However, screenees and registry participants also had the option of reporting data telephonically. Registry eligibility criteria included being 21 to 79 years of age at enrollment, having CLBP (≥3 months), having a physician (ie, allopathic or osteopathic physician) who provided usual care for CLBP, and sufficient English language proficiency to complete case report forms independently or with staff assistance. Screenees who reported being pregnant or residing in institutional facilities were excluded from the registry. The eligibility criteria were met in 4671 (55.0%) of the 8491 screening encounters during the study period and 1501 participants were enrolled. All study data were self-reported by registry participants at enrollment and quarterly encounters for up to 12 months. Data were not independently corroborated by physicians or medical records. Further information about PRECISION eligibility criteria and data collection is available at ClinicalTrials.gov.12 The research was approved by the North Texas Regional Institutional Review Board (protocol 2015-169), and all participants provided informed consent before contributing data. This study is reported following the STROBE guidelines.13
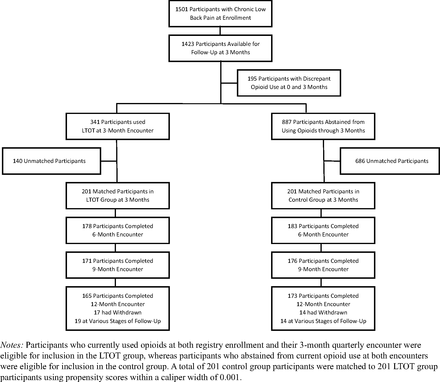
Participant flow through the study. Abbreviation: LTOT, long-term opioid therapy.
Measurement of Long-Term Opioid Therapy
Current opioid use was measured at registry enrollment by adapting the Minimum Dataset item recommended by NIH,14 which queries participants about their use of opioid painkillers for low back pain. The item provided prompts for specific medications, including generic and brand names of commonly used opioids. Current opioid use at registry enrollment may have entailed either new-onset use or unspecified longer-term use before enrollment. Long-term opioid therapy was defined as opioid use for >90 days,6 and established by reporting current opioid use at both registry enrollment and the 3-month encounter. Participants who did not report current opioid use at both registry enrollment and the 3-month encounter were considered opioid abstainers (ie, controls). Participants who reported discrepant current opioid use at these encounters were not eligible for inclusion in the study.
Participants also reported more comprehensive drug information at registry enrollment using open-ended items for name, dose, and daily frequency of administration for up to 12 medications for low back pain or other medical problems. The daily MME dosage for LTOT users was computed using data provided at registry enrollment for opioid class, dose, and daily frequency of administration, with conversion factors recommended by CDC.4
Propensity Score Matching
The LTOT users and abstainers were matched on propensity scores computed with a logistic regression model that included participant characteristics at registry enrollment pertaining to demography (age, gender, race), psychological factors (pain catastrophizing, pain self-efficacy), low back pain and related factors (duration of low back pain, presence of chronic widespread pain, work loss ≥1 month, receipt of disability or workers’ compensation benefits, law suits or legal claims, prior low back surgery, health-related quality of life), and clinical status (low back pain intensity, back-related disability, pain impact). These variables were measured using elements of the Minimum Dataset14 supplemented by the Pain Catastrophizing Scale,15 Pain Self-Efficacy Questionnaire,16 Patient-Reported Outcomes Measurement Information System (PROMIS),17 and other registry-specific instruments.12 All factors entered into the model were dichotomous or categorical variables, except health-related quality of life, low back pain intensity, back-related disability, and pain impact. Long-term opioid therapy users and abstainers were matched within a caliper width of 0.001 to ensure that no standardized difference between treatment groups was >0.10, which may represent a threshold for a meaningful imbalance in a given covariate.18
Outcome Measures
Primary outcomes included low back pain intensity, back-related disability, and pain impact. A numerical rating scale from 0 to 10 measured average low back pain intensity within 7 days before each encounter. Back-related disability was measured with the Roland-Morris Disability Questionnaire, which yielded values that reflected the participant’s status on the encounter date and ranged from 0 (no disability) to 24 (greatest disability).19 Pain impact was measured using low back pain intensity and 8 items derived from PROMIS.17,20 These included 4 items in each of 2 PROMIS scales involving physical function and pain interference with activities within 7 days before an encounter. Pain impact ranged from 8 to 50, with higher values representing worse outcomes. Secondary outcomes involved achievement of a minimally important change (MIC) in each primary outcome. These were defined as reductions of 1 point on the numerical rating scale for low back pain intensity,21 2 points on the Roland-Morris Disability Questionnaire,21 and 7.5 points on the pain impact scale.20 Outcome measures were collected at all completed quarterly encounters up to 12 months after registry enrollment. Participants who missed 2 consecutive quarterly encounters were withdrawn from the registry. However, available data for all withdrawn and in-progress participants before 12 months were retained and analyzed.
Statistical Analysis
Descriptive statistics were used to compare the baseline characteristics of participants in each treatment group at enrollment, including standardized differences to assess adequacy of propensity-score matching. The registry’s digital research platform and electronic data capture system precluded missing item responses on outcome measures at any completed encounter. However, participants may have reported ambiguous opioid names, doses, or daily frequencies of administration in open-ended fields, such as when they used pain-contingent (ie, “prn”) opioid dosing rather than fixed time-scheduled dosing.22 The resolution of ambiguous opioid names, doses, or daily frequencies of administration was generally performed by consensus among the investigators. This was facilitated by RxNorm, a naming system for generic and branded drugs developed by the National Library of Medicine to provide normalized names and unique identifiers for medicines,23 which has been used in the development of the medications component of diagnostic decision support systems.24 However, such resolution was not possible if participants reported prn dosing. In such cases, as in prior research involving opioid use among patients with chronic pain, multiple imputation was used to estimate missing drug data by incorporating key analytic variables and other variables potentially associated with the missing data.25,26 Our imputation model for missing daily MME dosage included low back pain intensity, back-related disability, and pain impact at registry enrollment as key analytic variables, and age and gender as other variables potentially associated with the missing data. One hundred imputations were run to minimize statistical power falloff for small effect sizes.27
In addition to overall analyses involving outcomes based on the LTOT groups established at 3 months using propensity-score matching, 4 other analyses were performed to assess continuity of opioid use and dose response. The first analysis measured the continuity of opioid use beyond the >90 days minimally needed to qualify for the LTOT group, and was based on the number of months of continuous opioid use, defined as 0, 3, 6, 9, or 12 months since enrollment. The second analysis measured dose response based on the total number of quarters during which opioids were used (not necessarily continuously used), ranging from 0 to 4 quarters over 12 months. The third analysis measured dose response based on daily MME dosages at registry enrollment. Daily MME dosages were classified as MMEs ≤ 30 (typical starting dosages for opioid-naïve patients), 30<MMEs < 50 (escalated opioid dosages for chronic pain), and MMEs ≥ 50 (dosages beyond which many patients may not experience benefit in pain or function).4 In each of these 3 analyses, analogous to intention-to-treat analyses in clinical trials, all participants were retained in their initial treatment group (LTOT or control) regardless of any subsequently reported treatment crossed over. Consequently, controls were classified as having no opioid exposure or dose in these 3 analyses. However, analogous to per-protocol analyses used in clinical trials to mitigate issues potentially pertaining to treatment crossover, the fourth analysis compared the subset of participants who were continuous opioid users versus continuous abstainers for all 12 months.
Generalized estimating equations (GEEs) were used to compare treatment groups on each primary outcome over 4 quarterly encounters (3, 6, 9, and 12 months) after LTOT and control group membership was established based on current opioid use and propensity-score matching. These analyses were performed using an autoregressive correlation matrix to compute maximum likelihood estimates for linear scale responses. To assess the possibility of misspecification of the GEE model or violation of its underlying assumptions,28,29 sensitivity analyses were performed using linear mixed methods with an unstructured correlation matrix. Cox proportional hazards regression was used to measure treatment group differences in time to achieve a MIC for each primary outcome.
Statistical power was estimated with the GLIMMPSE software for repeated measures.30 This was based on the primary low back pain intensity outcome because it is almost universally measured in CLBP trials. Forty-two high-quality trials of opioids versus placebo found a standardized difference of 0.69 favoring opioids on a 0 to 10 scale for low back pain intensity.10 The standard deviation of this scale was estimated to be 2.33.31 Assuming low back pain intensity base correlation = 0.6 with decay rate = 0.2 and inflation for 10% missing encounters over 12 months, 370 participants (185 in each treatment group) were needed for ≥0.90 statistical power. All data were managed and analyzed using the IBM SPSS Statistics Software (Version 29). Hypotheses were assessed at the 0.05 level of statistical significance, using 2-sided testing for all analyses (Appendix Table 1).
Results
Participant Flow Through the Study
A total of 341 (22.7%) and 887 (59.1%), respectively, of 1501 registry participants were eligible for the LTOT and control groups (Figure 1). There were 201 LTOT users and 201 matched controls included in the study (Table 1). The mean age of matched participants was 55.4 years (S.D., 11.9 years), and 297 (73.9%) were female. There were no significant baseline differences between treatment groups, and none of the standardized differences between them exceeded 0.10. In the LTOT group, 165 (82.1%) participants completed the 12-month encounter, 19 (9.5%) were still in progress at 6 or 9 months, and 17 (8.5%) had been withdrawn. Correspondingly, in the control group, 173 (86.1%) participants completed the 12-month encounter, 14 (7.0%) were in progress at 6 or 9 months, and 14 (7.0%) had been withdrawn (P = .54).
Baseline Characteristics of Study Participants by Treatment Groupa
Opioid Use
Among participants in the LTOT group, 87 (43.3%) used hydrocodone, 63 (31.3%) used tramadol, 38 (18.9%) used oxycodone, and the remainder used other opioids at registry enrollment. A total of 43 (21.4%), 23 (11.4%), 16 (8.0%), and 119 (59.2%) participants, respectively, used opioids continuously for durations of 3, 6, 9, or 12 months. There were 25 (12.4%), 23 (11.4%), 34 (16.9%), and 119 (59.2%) participants, respectively, who used opioids for 1, 2, 3, or 4 quarters. In the control group, there were 31 (15.4%), 16 (8.0%), 22 (10.9%), and 132 (65.7%) participants, respectively, who abstained from opioids for 3, 6, 9, or 12 months. Thirty-nine (19.4%) participants in the LTOT group crossed over to a period of opioid abstinence during follow-up, compared with 37 (18.4%) participants in the control group who crossed over to opioid use (P = .80). There were 78 (38.8%) participants in the LTOT group who required imputation of daily MME dosages. Overall, the mean daily MME dosage was 36.7 (95% CI, 32.8 to 40.7). Daily opioid dosages ≤30MMEs were used by 100 (49.8%) participants in the LTOT group; 30<MMEs < 50 were used by 66 (32.8%) participants; and MMEs ≥ 50 were used by 35 (17.4%) participants. The latter included 11 (5.5%) participants who used daily MMEs ≥ 90.
Primary Outcomes
There were no differences between the LTOT and control groups in any primary outcome during 12 months of follow-up (Figure 2). Similar results were observed in analyses for continuity of opioid use and dose response. (Table 2). However, among participants who continuously used or abstained from opioids for 12 months, the LTOT group reported greater pain intensity (mean, 6.13, 95% CI, 5.81-6.45 vs 5.58, 95% CI, 5.27-5.89; P = .02) and pain impact (mean, 32.83, 95% CI, 31.39-34.26 vs 29.56, 95% CI, 28.08 to 31.03; P = .002) than the control group. Sensitivity analyses involving linear mixed methods and an unstructured correlation matrix yielded results similar to those observed in the main analyses (Appendix Table 2).
Primary outcomes over time. Abbreviation: LTOT, long-term opioid therapy.
Primary Outcomes Over Timea
Secondary Outcomes
Overall, 132 (65.7%) participants in the LTOT group achieved a MIC in low back pain intensity during 12 months of follow-up versus 139 (69.2%) participants in the control group (P = .46). Respectively, in the LTOT and control groups, 106 (52.7%) versus 111 (55.2%) participants achieved a MIC in back-related disability (P = .62), and 40 (19.9%) vs 56 (27.9%) participants achieved a MIC in pain impact (P = .06). There were no differences between treatment groups in time to achieve a MIC (Table 3). Similar results were observed in analyses for continuity of opioid use and dose response. However, among participants who continuously used or abstained from opioids for 12 months, LTOT users reported lower likelihood of a MIC in pain impact (HR, 0.60; 95% CI, 0.37-0.98; P = .04).
Secondary Outcomes Over Timea
Discussion
A clinical review of CLBP management cited the limitation of clinical trials having only short or intermediate duration.32 Our study found that CLBP treatment involving LTOT over 12 months is not more effective in improving low back pain intensity, back-related disability, or pain impact than treatment without opioids. These findings were corroborated in analyses that measured both continuity of opioid use and dose response, including continuous opioid use versus abstinence for 12 months. In the latter analyses, LTOT users reported worse outcomes for low back pain intensity and pain impact, and lower likelihood of a MIC in pain impact than control participants. Thus, our findings further underscore the importance of carefully weighing the potentially limited benefits of opioids against their known risks among patients with CLBP.4 When patients have been using opioids for long durations (eg, ≥12 months), dosages that are tapered by 10% per month or slower will most likely be better tolerated than more rapid tapers.33 By contrast, discontinuation or rapid reduction of high-dose LTOT has been associated with increased risk of opioid overdose and opioid use disorder.34
Although randomized controlled trials are powerful tools to assess therapeutic benefit while accounting for unknown confounders, observational registries that collect standardized data from patients in a variety of settings may offset the complexity, expense, and time required for recruitment and long-term follow-up in clinical trials.35 Our use of a national pain research registry to conduct a retrospective cohort study with propensity score matching enabled us to supplement current evidence on LTOT from randomized controlled trials in several ways. First, it provided data that are more generalizable to real-world experiences of patients with CLBP than would be acquired in a clinical trial. Second, it conducted follow-up for 12 months, which is longer than previously reported in clinical trials that focused on CLBP in general populations. Our research design also averted ethical concerns with randomizing patients to LTOT for up to 12 months when benefits of such therapy are questionable and associated risks are well known. Third, propensity-score matching was used to help mitigate confounding by indication36 and involved psychological and clinical variables that are not routinely measured in clinical trials involving CLBP, including pain catastrophizing, pain self-efficacy, widespread pain, and health-related quality of life. Fourth, participants were also matched on wage replacement benefits and litigation, which likely influence treatment effects but are infrequently measured in clinical trials.10
There were several limitations of our study. First, although standardized differences between treatment groups were within suggested limits after propensity score-matching, other unmatched variables may have contributed to residual confounding owing to lack of randomization. Second, comprehensive data on opioid name, dose, and daily frequency of administration were collected by the registry at enrollment to compute daily MME dosages,12 but opioid use at the 3-, 6-, and 9-month encounters was measured using only the single item on the NIH Minimum Dataset.14 That item queried about current use of opioids, but not drug name, dose, or daily frequency of administration. It also did not inquire about opioid use during interim months between each quarterly encounter. Third, about 39% of participants in the LTOT group required imputation of daily MME dosages primarily because they used prn opioid dosing and the registry does not ask participants to keep daily medication diaries. Fourth, treatment crossover occurred in about 19% of participants in both the LTOT and control groups. To help address this limitation, we conducted analyses involving only participants with continuous opioid use or abstinence for 12 months. Although these analyses corroborated lack of LTOT effectiveness, and further demonstrated worse outcomes in low back pain intensity and pain impact with LTOT, the subset of participants in these analyses may no longer have been adequately matched on propensity scores. Fifth, the registry does not collect data on opioid use in the months before enrollment. Thus, it is possible that some LTOT users may have been longer-term users than reported herein. Finally, in this pragmatic study, current opioid use was assessed at quarterly encounters as part of usual care for CLBP. However, it was not possible to measure potential statistical interaction between opioid use and other medications or nonpharmacological treatments (eg, physical therapy and complementary or alternative medicine therapies) used for low back pain throughout the study.
In summary, our study findings indicate that CLBP treatment involving LTOT for up to 12 months is not more effective in improving low back pain intensity, back-related disability, or pain impact than treatment without opioids. Clinicians should carefully weigh the potentially limited benefits of opioids against known risks when prescribing them for CLBP and consider slowly tapering opioid dosage in patients using them for long durations of time.
Appendix.
Overview of Analyses, Statistical Methods, and Scale and Timing of Measuresa
Sensitivity Analyses for Primary Outcomes over Timea
Web landing page advertised on social media to recruit participants for the pain registry for epidemiological, clinical, and interventional studies and innovation. Respondents completed the screening questionnaire by clicking on the “Participate” link (photo credit, Aleksandra Suz/Shutterstock.com).
Notes
This article was externally peer reviewed.
Funding: None.
Conflicts of interest: None.
To see this article online, please go to: http://jabfm.org/content/37/1/59.full.
- Received for publication April 11, 2023.
- Revision received July 25, 2023.
- Accepted for publication August 3, 2023.