Abstract
Background: Millions of children have tested positive for SARS-CoV-2, and over 1000 children have died in the US. However, vaccination rates for children 5 to 11 years old are low.
Methods: Starting in August 2020, we conducted a prospective SARS-CoV-2 household surveillance study in Spanish and English-speaking households in New York City and Utah. From October 21 to 25, 2021, we asked caregivers about their likelihood of getting COVID-19 vaccine for their child, and reasons that they might or might not vaccinate that child. We compared intent to vaccinate by site, demographic characteristics, SARS-CoV-2 infection detected by study surveillance, and parents’ COVID-19 vaccination status using Chi-square tests and a multivariable logistic regression model, accounting for within-household clustering.
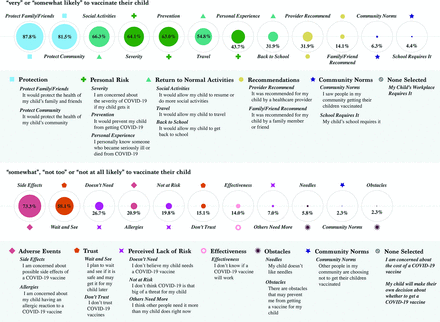
Results: Among parents or caregivers of 309 children (0 to 11 years) in 172 households, 87% were very or somewhat likely to intend to vaccinate their child. The most prevalent reasons for intending to vaccinate were to protect family and friends and the community; individual prevention was mentioned less often. The most prevalent reasons for not intending to vaccinate were side effect concerns and wanting to wait and see.
In multivariable analysis, parents had much lower odds of intending to vaccinate if someone in the household had tested SARS-CoV-2-positive during the study (adjusted odds ratio = 0.09; 95% confidence interval, 0.03-0.3).
Conclusion: This study highlighted several themes for clinicians and public health officials to consider including the importance and safety of vaccination for this age-group even if infected previously, and the benefits of vaccination to protect family, friends, and community.
- Caregivers
- Child
- Chi-Square Test
- COVID-19 Vaccines
- Logistic Models
- New York City
- Parents
- Prospective Studies
- Public Health
- SARS-CoV-2
- Utah
Introduction
Over 13.8 million children aged <18 years have tested positive for SARS-CoV-2 in the United States1 with more than 1290 reported pediatric deaths.2 Although the Food and Drug Administration (FDA) approved an Emergency Use Authorization for the COVID-19 vaccine for 5 to 11 year-olds on October 29, 2021, only 36% of children in this age-group were vaccinated as of June 2022, a number that is only slowly increasing.1 COVID-19 vaccines for children aged <5 years have just recently been authorized. Understanding factors affecting parents’ willingness to vaccinate their children is critical to promoting uptake.
Methods
We previously reported the methods and findings of a SARS-CoV-2 household surveillance study in Spanish and English-speaking households in New York City and Utah.3 During October 21 to 25, 2021 immediately before the FDA meeting, we conducted a short survey asking the reporting caregiver of each child aged 0 to 11 years about their intent to vaccinate that child against COVID-19 (very, somewhat, not too or not at all likely), and reasons that they might (reported “very” or “somewhat likely”) or might not (“somewhat,” “not too” or “not at all likely”). Response options about reasons to vaccinate or not vaccinate were adapted from CDC national internet panel surveys that were field tested and have been used to assess attitudes to COVID-19 vaccination periodically during the pandemic. Only 1 caregiver reported for each child, but 1 could report for multiple children each separately. We compared intent to vaccinate (very/somewhat likely vs not too/not at all likely) by site, demographic characteristics, SARS-CoV-2 infection detected by study surveillance among household members during the study period, and parents’ COVID-19 vaccination status using Chi square tests. Samples were not reweighted in either the main study or this analysis. Variables with a univariate p value <0.1 were entered into a multivariable logistic regression model. Generalized estimating equations assuming working independence with a robust variance estimator addressed correlation from within-household clustering. The University of Utah Institutional Review Board (IRB) was the central IRB and approved the study.
Results
Among respondents for 309 children (305 parents, 2 grandmothers, 2 aunts, response rate 78%) in 172 households, 87% were very or somewhat likely to vaccinate their child (Table 1). The most prevalent reasons for vaccinating were to protect family and friends and the community; individual prevention was endorsed less often. The most prevalent reasons for not vaccinating were side effect concerns and wanting to wait and see (Figure 1). These were the top themes regardless of site or age (0 to 4 years and 5 to 11 years).
Reasons respondent parent would chose to vaccinate or not to vaccinate their child aged 0 to 11 years.
Characteristics of Study Population and Associations with Parental Intentions for COVID-19 Vaccination of Their Child (N = 309), Respondents from 172 Households
In multivariable analysis, parents had much lower odds of intending to vaccinate if someone in the household had tested SARS-CoV-2-positive during the study (adjusted odds ratio [aOR] 0.09; 95% confidence interval, 0.03 to 0.3) (Table 1). Higher household income aOR 13.9 (1.2 to 165.8) and parental COVID-19 vaccine receipt aOR 12.3 (2.1 to 73.2) were independently associated with vaccine intentions, but not child’s age, childcare/school attendance outside the home, parental education level, or other selected demographic factors (Table 1). Trends were similar when chi squares were stratified by age-group and by site.
Discussion
In this study, most parents intended to vaccinate their children aged 0 to 11 years once vaccine was available. This study included a convenience sample of a relatively small number of households, and persons of certain racial and ethnic backgrounds, those who were low-income and those who were uninsured persons were under-represented. Thus, findings may not be generalizable to all segments of the US population. However, findings suggest several themes for clinicians and public health officials to consider when thinking about communication strategies as part of multi-level interventions. First, it may be important to underscore to families the importance of vaccination even if household members have previously had SARS-CoV-2 infection.4 Families may also need reassurance about vaccine safety after prior infection. Second, for this younger age-group, the benefits of vaccination to protect family, friends and community may play a particularly important role, even more than personal protection, in motivating parents to vaccinate their child. Addressing concerns about vaccine side effects also remains important. Third, low-income households may benefit from additional outreach efforts to provide information about COVID-19 vaccines for children. Finally, while parental vaccination status was associated with vaccine intention for children,5 over 50% of unvaccinated parents reported intention to vaccinate their child, demonstrating the importance of offering COVID-19 vaccine, regardless of parental vaccination status.
Acknowledgments
The authors acknowledge the families that participated in the C-HEART cohort, the REDCap data platform, and the following people for contributing to the study: Melissa A. Rolfes, PhD, MPH from the Centers for Disease Control and Prevention; Priyam Thind, MPH, Maria Castro, MS, Alisha Sarakki, MPH, and John Paul Harris from Columbia University; Jonah M. Stockwell (figure creation); Emily Hacker, BS, Jacob Anderson, Halle Fiagle, and Kathryn Graham from University of Utah; and Zuha Jeddy, MPH, David Izrael, MS, Utsav Kattel, BSc, Kim Altunkaynak, MS, Danielle Rentz Hunt, PhD, and Parker Malek, BS, from Abt Associates.
Notes
This article was externally peer reviewed.
Funding: This study was funded by the U.S. Centers for Disease Control and Prevention through Contract # 75D30120C08150 with Abt Associates. The funder did participate in the work. Findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention.
Conflict of interest: Dr Porucznik reports personal fees from McKesson Corporation outside the submitted work. The other authors have no relevant conflicts of interest to disclose.
To see this article online, please go to: http://jabfm.org/content/35/6/1174.full.
- Received for publication February 16, 2022.
- Revision received July 13, 2022.
- Accepted for publication July 18, 2022.