Abstract
Oropharyngeal squamous cell carcinoma (OPSCC) has historically been attributable to tobacco and alcohol exposure and saw a decline in incidence after societal norms shifted away from smoking. In recent decades, this disease has had a re-emergence due to human papillomavirus (HPV) infection, now surpassing cervical cancer as the number 1 cause of HPV-related cancer in the United States. HPV-positive OPSCC differs from HPV-negative disease in epidemiology, prognosis, treatment, and prevention. Additionally, there is a deficit in awareness of the causal relationship between HPV and OPSCC. This, coupled with low vaccination rates, puts primary care providers in a unique position to play a vital role in prevention and early diagnosis. In this review, we highlight the epidemiology, screening, patient presentation, diagnosis, prognosis, and prevention of HPV-positive OPSCC, with a focus on the primary care provider's role.
- Immunization
- Otolaryngology
- Papillomavirus Vaccines
- Patient Care Team
- Primary Health Care
- Sexually Transmitted Diseases
- Squamous Cell Carcinoma of Head and Neck
Introduction
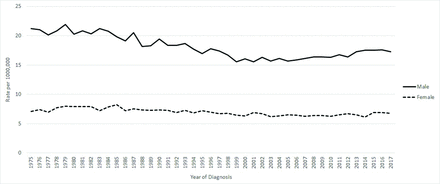
Oropharyngeal squamous cell carcinoma (OPSCC) has historically been attributable to tobacco exposure, seeing a decline in incidence in the 1980s (Figure 1)1 after societal norms shifted away from smoking. However, in recent decades this disease has had a re-emergence due to human papillomavirus (HPV) infection, now surpassing cervical cancer as the number 1 cause of HPV-related cancer in the United States.2 Compared with HPV-negative OPSCC, the disease course of HPV-positive OPSCC portends a more favorable prognosis, with significantly increased progression free survival.3 This is particularly true when appropriate therapy is provided early, highlighting the importance of early detection and treatment to reduce the morbidity associated with late-stage interventions. Although treatment interventions are improving, the majority of these cases are attributable to HPV 16, 18, 31, and 33; all infections preventable by vaccination.
SEER Age-Adjusted Trends in Oral Cavity and Pharynx Cancer, 1975 to 2017.1 Results from cancer incidence data from population-based cancer registries covering approximately 34.6% of the US population.
This puts primary care providers (PCPs) in a unique position to play a vital role in prevention, screening, and early diagnosis. In this review, we highlight the epidemiology, screening, patient presentation, diagnosis, prognosis, and prevention of HPV-positive OPSCC, with a PCP-focused approach.
Epidemiology
OPSCC has historically been a disease affecting older male smokers. As the rates of tobacco consumption have declined in the United States, there has been a decreased incidence of many head and neck cancers. HPV-related head and neck cancers, however, have not followed this overall trend, with multiple sources demonstrating a rise in incidence over the last 2 decades.4⇓–6 Specifically, the percentage of HPV-related cancers has jumped from 41%, before 2000, to 72% between 2005 and 2009 (Figure 2).7 While previous literature estimated the incidence of HPV-related OPSCC would surpass HPV-related cervical cancer by 2020,8,9 data collected by the Centers for Disease Control and Prevention (CDC) have suggested that the total incidence has already surpassed HPV-related cervical cancer well before this time frame.2
Human Papillomavirus (HPV) Prevalence Among Patients With Oropharyngeal Cancer as Reported in A Systematic Review and Meta-Analysis of Studies Published Between 1966 and 2010.7
While marijuana exposure has been shown to be an independent risk factor for the development of HPV-positive head and neck cancer,10 the primary reason for the increase in OPSCC is related to an overall rise in high-risk sexual practices. More than 90% of oral HPV infections are related to sexual activity, with oral sex being the largest predisposing factor.11,12 Furthermore, patients impacted by HPV-positive OPSCC are generally younger than their HPV-negative counterparts and overwhelmingly white males—typically between the ages of 40 and 55.13
Screening and Presentation
Unlike cervical cancer, which uses Papanicolaou cytology screening to assess precancerous lesions of the cervix, there are currently no practical screening tools available for HPV-positive OPSCC screening.12 Several techniques are under investigation, but each has limitations that preclude its use for population-level screening (Table 1). Furthermore, validated sexual history screening questionnaires have not been developed to capture patients most at risk.
Proposed HPV Screening Tools12
Without a validated screening test or questionnaire to detect patients with or most at risk for HPV-positive OPSCC, it is imperative that first-line health care providers (PCPs and dentists) remain cognizant of the current epidemiologic factors discussed above. This information, coupled with knowledge and awareness of common clinical presentations, can be greatly beneficial to patients. A recent retrospective analysis of 207 patients with OPSCC found that patients with HPV-positive OPSCC were most likely to present with a neck mass (56%), sore throat (11%), or oral mass (11%).14 Furthermore most HPV-positive patients in this study presented at a tumor, node, metastasis stage of IVa (57%), with the tonsil (60%) and base of tongue (40%) being the most common primary tumor site.14
If a patient presents to the office with a history suspicious for OPSCC, it is recommended that providers conduct a formal head and neck examination that includes inspection of the oral cavity, palatine tonsils, base of tongue, and cervical lymph nodes. An external light source and tongue blade are important for optimum visualization of the oral cavity and oropharynx for asymmetries or lesions. A mirror may be used to inspect the vallecula. Thorough palpation of the tonsillar fossae and tongue base can identify induration, ulceration, or swelling.15
Prevention
The majority of HPV-positive OPSCCs are caused by HPV 16 with minor contributions from 18, 31, and 33. In 2006, the first HPV vaccine, Gardasil 4, was approved for prevention of cervical cancer and covered HPV 6, 11, 16, and 18. The latest vaccine, Gardasil 9, covers HPV strains 6, 11, 16, 18, 31, 33, 45, 52, and 58 and therefore protects against the most carcinogenic strains of HPV. It has gained US Food and Drug Administration (FDA) approval for prevention of cervical, vulvar, vaginal, anal, oropharyngeal, and other head and neck cancers16 and is administered in either 2 or 3 doses depending on the age of the patient at the time of the first dose (Table 2). The primary target group for the vaccine is children over the age of 9, ideally before the onset of sexual activity; however, Gardasil 9 has been FDA approved for both men and women up to the age of 45.17 Despite this expanded coverage, the CDC does not currently recommend routine HPV vaccination for patients above the age of 26 but rather shared clinical decision-making on a case-by-case basis.18,19 Because of this, insurance may not always cover the vaccine in this extended age group.
Dosing Schedule of Gardasil 9 Vaccine as Recommended by Manufacturer16
Despite the wide availability of HPV vaccines in the United States, many providers are unaware of the link between HPV and OPSCC. A 2017 study reported that only 16% of pediatricians were aware of the link between OPSCC and HPV, and only 46% had knowledge that HPV-related oropharyngeal cancer incidence was increasing in the United States.20 What is more alarming is that a 2019 national vaccination study found that among adolescents between 13 and 17 years old, only 54% were up to date with the recommended HPV vaccination series.21 Males (52%) were shown to have a slightly lower vaccination rate than females (57%).
The lack of awareness and low vaccination rate highlights the paramount role that PCPs play in disease prevention. While the vaccine is targeted for virginal adolescents, a recent study of men between the ages of 27 and 45 found that the immune response in this population was comparable to that of younger men ages 16 to 26.22 Armed with this information, PCPs can increase HPV vaccination rates and greatly reduce the morbidity and mortality associated with OPSCC.
In addition, oro-genital sex is the most important risk factor for developing HPV-positive OPSCC. While studies have shown that properly utilized barrier methods of protection (condoms and dental dams) can lower the risk of contracting HPV,23 in practice, they have poor adoption and user compliance for oral sex, especially among young adults.24 Thus, although it is prudent to suggest barrier use for patients who are receptive, it should not serve as an alternative to vaccination, especially for high-risk individuals with multiple sexual partners.
Lastly, smoking is a well-known risk factor for HPV-negative OPSCC but may also be associated with increased risk of HPV-positive OPSCC. A recent study demonstrated that smoking prolongs HPV infections in the oral cavity,25 suggesting it could play a role in the pathogenesis of HPV-related OPSCC. This provides more evidence to support smoking cessation for patients, especially those at high risk or who have not been vaccinated.
Diagnosis
While it is important to know the common clinical presentation, a high index of suspicion is necessary when patients have presentations concerning for a neck mass. If a lesion is identified, suspected but not visualized, or the patient endorses troublesome symptoms, a low threshold for referral to an otolaryngologist for further workup is necessary. Workup includes imaging and visualization of primary sites via endoscopy, although OPSCC can only be diagnosed via biopsy of a primary or metastatic lesion. Following the biopsy, tumor samples will undergo either polymerase chain reaction amplification of HPV DNA or in situ hybridization to determine if it is HPV-positive or HPV-negative.
Treatment
Trends in the treatment of patients with OPSCC are constantly evolving. Historically, large open resections were the predominant treatment approach for these patients. Due to severe functional morbidity and high complication rates associated with these invasive procedures, chemoradiation (CRT) became the preferred treatment option in the 1990s.26 CRT provides equivalent overall survival for patients with OPSCC with less functional morbidity when compared with open surgery. Unfortunately, as CRT became more widely adopted, it was clear that it had its own toxicity profile, including dysphagia, osteoradionecrosis, mucositis, and xerostomia, all which have profound effects on the quality of life for cancer survivors.27 With the rise in HPV-related OPSCC, and a much younger population being affected, it was imperative to consider the long-term consequences of the treatment regimen. This in part is why treatment again shifted toward less invasive surgical approaches such as transoral robotic surgery (TORS). TORS has been shown to have equivalent outcomes as CRT, and it may allow for improved long-term outcomes from CRT de-escalation.28 Currently, there is literature to support improved quality of life in patients treated with TORS,29,30 although the subject at the moment is controversial. Recently the ORATOR trial, a phase 2 randomized study demonstrated that patients undergoing TORS actually had worse swallowing-related quality of life scores 1 year after treatment, compared with the radiotherapy-treated group.31
Follow-up
Follow-up for these patients is complex, often requiring multiple specialists to treat their disease such as medical oncologists, head and neck radiation oncologists, otolaryngologists, speech language pathologists, and nutritionists. The PCP is not only essential in ensuring that the patient is following up appropriately with specialist services but is also vital in managing any other illnesses or comorbidities that would significantly impact the prognosis. Follow-up with otolaryngology is generally extensive, and patients are seen for many years following treatment for tumor surveillance.
Conclusions
HPV-positive OPSCC is the most common HPV-related malignancy in the United States and affects a different demographic of individuals than HPV-negative OPSCC. PCPs are crucial in primary prevention of disease through vaccination and can dramatically improve patient prognosis through early detection. By increasing awareness of the distinctions between HPV-negative and HPV-positive OPSCC among PCPs, we could decrease the burden of illness among the population.
Notes
This article was externally peer reviewed.
Funding: None.
Conflict of interest: None.
To see this article online, please go to: http://jabfm.org/content/34/4/832.full.
- Received for publication November 11, 2020.
- Revision received March 3, 2021.
- Accepted for publication March 11, 2021.