Abstract
Background: Pharmaceutical interaction in US residencies is common. This study explores the extent and type of learner interactions in US family medicine residencies with the pharmaceutical industry and compares interactions from 2008, 2013, and 2019.
Methods: We surveyed program directors of 628 family medicine residencies with 8 questions using the 2019 Council of Academic Family Medicine Educational Research Alliance Survey and compared the responses to 2008 and 2013 results.
Results: The survey response rate was 39%; 81% of responding residencies did not allow food or gifts, 86% did not allow drug samples, 84% did not allow industry to interact with medical students or residents, and 81% did not allow industry-sponsored residency activities. These numbers were statistically significantly higher than both 2008 and 2013. In 2019, 151 responding programs (64%) were pharma-free, that is, they answered “No” to all 4 questions about interactions. Pharma-free residencies were increased in 2019 compared with 26% in 2008% and 49% in 2013. University-based family medicine programs were more likely to be pharma-free. Only 21% of responding programs had a formal curriculum that explores the interaction between physicians and the pharmaceutical industry. Factors cited for decreasing interaction included: institutional policy, ethical concerns, faculty input, and local response to national legislation.
Conclusions: Interaction between trainees in US family medicine residencies and the pharmaceutical industry continued to decrease. A changing national legislative landscape combined with institutional policies and concerns about industry influence on prescribing habits may be important factors driving the limiting of interactions.
- Conflict of interest
- Drug Industry
- Family Medicine
- Graduate Medical Education
- Internship and Residency
- Marketing
- Organizational Policy
- Pharmaceutical Economics
- Surveys and Questionnaires
Introduction
Pharmaceutical industry interactions are common in medical schools and residency training programs.1 Many residents (54% in 1 recent survey) have accepted gifts or food.2 Residency programs in multiple specialties allow food and other gifts, as well as industry sponsorship of lectures, journal clubs, and other events.2⇓⇓–5 A 2011 survey of medical students and residents found that 49% of residents had met with pharmaceutical representatives, and 36% had attended industry-sponsored lectures.2 The more contact learners have with industry, the more positively they felt about industry interactions.6,7
Industry gifts to physicians are common8⇓–10 and interactions with the pharmaceutical industry affect physician attitudes and prescribing behavior.7,11⇓⇓⇓⇓⇓⇓–18
Prescribing habits are formed during medical training. Nonrational prescribing is associated with trainee exposure to industry promotion.6,7 Access to drug samples, an important form of promotion, also changes physician prescribing patterns.19⇓⇓–22
There are few studies from the past 15 years on the quantity of pharmaceutical interaction with residents. A survey of US internal medicine residency program directors in 2006 to 2007 found that 56% accepted support from industry.3 A survey of 122 ophthalmology residency programs in the United States found that drug reps visited 87% of programs at least monthly; most respondents had accepted gifts.5 In a 2009 study of surgical residency training programs about two thirds reported industry-sponsored meals.4 In colorectal surgery programs in 2013, 69% of program directors and 60% of other faculty received 1 or more payments in the last half of 2013. Fourty-nine (93%) programs had surgeons who received funding.23
Some medical schools and residency programs teach formal curricula on physician-pharmaceutical industry interactions.24 In 2013, 4 in 10 family medicine residency programs had such a curriculum.25 Curricula are more common in residencies that allowed interaction with industry (52%) compared with the residencies that did not allow interaction with industry (30%).25
There is evidence that physician-industry interaction in the United States is decreasing. A 2009 survey showed that physician interactions with industry had decreased since 2004, although 84% of physicians still had some relationship.10 From 2006 to 2009, numerous experts and national organizations called for limiting industry involvement in training programs.26⇓⇓–29 The Sunshine Provision of the 2010 Affordable Care Act required the pharmaceutical industry, starting in 2014, to report payments and gifts to teaching hospitals and physicians. Required reporting may limit interactions and gifts.15,30
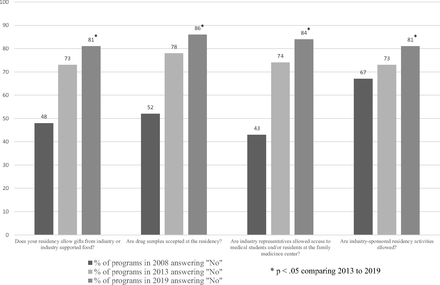
Revised standards addressing commercial support of continuing medical education may also be a factor in changing policy and perception, although there is ongoing controversy about whether these revisions decreased financial conflicts of interest.31 We published 2 surveys of pharmaceutical industry interactions in family medicine residencies that showed that industry interaction decreased substantially between 2008 and 2013.32–33 Residency programs declined more gifts or industry-sponsored food (48% to 73%) and increased refusal of sample medications (52% to 78%). Fewer programs allowed industry representatives to have access to learners (43% to 74%). Residency programs that forbade industry-sponsored activities remained stable over the 5-year time period (67% vs 73%).
In 2019, we surveyed family medicine residency program directors to determine whether the trend of decreasing industry interactions has continued since 2013 and to assess changes in pharmaceutical industry interaction.
Methods
The 2019 survey of program directors of US family medicine residencies was conducted as part of a larger omnibus survey, the Council of Academic Family Medicine Educational Research Alliance (CERA) Program Director Survey. The methodology of the CERA Program Director Survey has previously been described in detail.34 The CERA steering committee, a group with expertise in survey creation and testing, evaluated questions for consistency with the overall subproject aim, readability, and existing evidence of reliability and validity. Pretesting was done on family medicine educators who were not part of the target population. The questions were modified following pretesting for flow, timing, and readability. The project was approved by the American Academy of Family Physicians Institutional Review Board in September 2019. Data were collected from September 2019 to November 2019.
The sampling frame for the survey was all Accrediting Council of Graduate Medical Education accredited US family medicine residency program directors, as identified by the Association of Family Medicine Residency Directors. E-mail invitations to participate were delivered with the survey using the online program, Survey Monkey. Six follow-up e-mails to encourage nonrespondents to participate were sent after the initial e-mail invitation. There were 668 program directors at the time of the survey. Forty had previously opted out or blocked Survey Monkey surveys. Therefore, the survey was emailed to 628 individuals.
The survey included 8 questions related to our analysis (see Table 1).
2019 CERA Program Director Survey Questions: Pharmaceutical Industry Interaction in US Family Medicine Residencies
Questions 1 to 4 were the same questions we asked in both the 2008 and 2013 surveys. We asked question 5 as a follow-up to our 2011 curriculum study,25 and questions 6 to 8 were new. We added a statement to question 3 regarding industry representatives: Note: This does not include access required for device training, such as Nexplanon. We heard from several program directors in the previous survey and subsequently that access is essential for optimal patient care and some were uncertain when answering this question. As our survey is largely regarding drug marketing, not devices, we felt adding the device statement would improve clarity.
Since our first survey,32 we’ve designated as “pharma-free” family medicine residencies that answered “No” to our original 4 questions. Pharma-free programs allow no industry gifts, food, samples, interaction with students or residents, or sponsorship of residency activities.
US family medicine residencies are categorized by program type: university based, community based, university affiliated, community-based nonaffiliated, and military. Programs self reported their program type.
Chi-squared testing employing contingency tables was used to compare results among years. A 2-tailed P < .05 was considered significant. The data were analyzed using IBM SPSS Statistics for Macintosh v 26.0, (IBM Analytics, Armonk, NY.)
Results
The overall response rate for the 2019 CERA Program Director survey was 39% (250/628). 237/628 (38%) answered our questions. The response rate in our 2013 CERA survey was 56% (251/445) and 62% (286/460) in our 2008 survey (a free-standing survey unrelated to CERA).
In 2019, 81% of responding residencies did not allow food or gifts, 86% did not allow drug samples, 84% did not allow industry to interact with medical students or residents, and 81% did not allow industry-sponsored residency activities. These numbers are significantly higher than both 2013 and 2008 (Figure 1) (P < . 05 in all 4 questions comparing 2019 to 2013).
2008, 2013, 2019 Comparison of responses of US family medicine residencies to a national survey concerning industry interactions with and access to trainees.
In 2019, 151 responding programs (64%) were pharma-free, that is, they answered “no” to all 4 questions about interactions. Pharma-free residencies increased from 26% in 2008% to 49% in 2013 (P = .001 comparing 2013 with 2019.)
University-based programs that responded to our survey (40/51, 78%) were more likely to be pharma-free than community-based university-affiliated programs (90/152, 59%) and community-based nonaffiliated programs (24/46, 52%). Three of 4 responding military programs are pharma-free (75%). (P = .02 comparing university-based to both types of community-based programs.)
Forty-nine responding residency programs (21%) have a formal curriculum that explores the interaction between physicians and the pharmaceutical industry. This is a sharp decrease from the 84 (40%) reported in our 2013 curriculum study (P < .001).25
Most responding program directors (n = 135, 57%) reported a decrease in pharmaceutical interaction in their programs within the past 5 years. Only 3 (1%) reported an increase, while 83 (35%) reported no change in interaction. Sixteen (7%) did not know.
The most common factors cited for a decrease in interaction were institutional policy (mentioned by 53), ethical concerns (mentioned by 46), faculty input (mentioned by 29), local response to national legislation (mentioned by 21), and resident input (mentioned by 9).
Discussion
Interaction with the pharmaceutical industry in family medicine residencies continues to decrease. Our 10-year follow-up study provides unique insights into changing industry relationships with residencies over time. No other studies have examined residencies in 1 specialty over 3 timepoints.
The decrease in interactions noted by responding programs was particularly marked between 2008 and 2013, but the trend continued from 2013 to 2019. Our data mirror changes among physicians in general. A 2017 survey showed that the percentage of physicians with any relationship with industry decreased from 84% in 2009 to 72% in 2017.10,35 In these studies, the number of physicians who receive drug samples decreased in 2017 compared with 2009 from 64% to 55% and the number of physicians receiving any food/beverage or tickets to cultural/sporting events decreased from 75% to 42%. An analysis of prescriptions and industry funding after the Sunshine Act showed a decrease in industry payments to physicians between 2014 and 2016.15 Medical student exposure to drug company interactions decreased from 2002 to 2013 and students became more skeptical of the benefit of pharmaceutical interaction over that time period.36
Changes in the health care landscape have been implemented since 2009, mostly notably the Sunshine Act, and numerous experts suggested limiting interaction.26⇓⇓–29 Some states have enacted strict gift bans or reporting rules.37 Until our study, there has been no recent research showing the impact of these changes on interaction in residency programs.
Most family medicine residencies (90%) are in a community setting.38 Most of the programs (80%) in a community setting are affiliated with or administered by a medical school, even if they are not located on the campus of an academic medical center.38 Academic medical centers and associated medical schools have been a focus of advocacy for eliminating pharmaceutical influence.26 A recent survey from the American Medical Student Association (AMSA) showed that only 4 medical schools banned pharmaceutical representatives from campus.39 It seems from our study that indeed academic medical centers are leading the way, although community programs are also majority pharma-free. Most medical students (65%) report private outpatient offices are the main source of exposure to industry marketing.36
Our surveys, unlike the AMSA scorecard, assessed practice, not policy. More than two thirds (70%) of medical schools now earn an “A” or “B” grade on the AMSA scorecard for their industry interaction policies.39 Policies may be ineffective: an important study shows that restrictive policies at medical schools did not make residents more likely to avoid industry interactions.40 This may be due to what has been called the “hidden curriculum,” which is the example set by mentors.41,42 The AMSA scorecard may have been a factor in ongoing change in academic medical centers.
According to our study, responding program directors believe that institutional policies have played an important role in decreased interaction. Other important factors include ethical concerns and input from faculty. National legislation is also a reason cited by many of our respondents.
The Accreditation Council of Graduate Medical Education suggests residency programs should educate residents about interaction with industry.1 Presence of formal curricula in family medicine residencies is decreasing based on our data from responding program directors We are not aware of any study in another specialty that measures rate of implementation of industry interaction curricula. Evans’ study of family medicine residencies found that curricula on physician- pharmaceutical industry interactions were more common in residencies that allowed industry interactions.25 Formal curricula vary widely and are not necessarily critical of industry tactics. A systematic review of 9 published curricula found inconsistencies in content, application, and evaluation methodology.24 It is plausible that residencies that allow industry interactions feel called on to have a countervailing influence. It seems unlikely, however, that a residency program that allows drug reps would inveigh against seeing drug reps. Programs that currently allow pharmaceutical interaction may find our data useful as they plan curriculum or future policy.
The drop in industry interactions we observed may be associated with the drop in formal curricula programs; residencies with few industry interactions may feel less need for a formal curriculum. Practice may be more important than policy. Pharmaceutical representatives may present inaccurate information. A study conducted in the United States, Canada, and France found that drug reps rarely mention serious adverse effects—even for drugs with black box warnings—and often promoted drugs off-label.43,44 False statements are common in conversations with drug reps.43 Pharmaceutical representatives are trained to pivot in discussion when the conversation is moving in a direction less favorable to the marketed drug.45 Howard Brody,46 a family physician and ethicist, argues that the time needed to find information to check the accuracy of pharmaceutical industry pitches far outweighs any benefit from the knowledge gained during an interaction.
Family medicine residency programs may have fewer relationships than other specialties, but this is difficult to ascertain. Degree of acceptance may vary by specialty, but direct comparisons are difficult because surveys of different specialties were done at different timepoints and using different methodologies. Further research is needed to compare interactions across specialties. Our 4 questions might be a useful approach to assess interaction in other residency specialties as there is no consensus on how to best measure pharmaceutical influence in residency programs.
Our study has several limitations. Our survey response rate of 38%, was lower than in previous years. This may be due to the expansion of family medicine residencies in the past 5 years. Our 2013 survey attempted to survey 445 directors while our 2019 survey attempted to survey 628 program directors. New program directors may be less familiar with the CERA survey and less likely to respond. It is possible that residencies that take money, gifts or samples from industry are less likely to respond. However, as the CERA omnibus survey covers multiple topics, bias to the topic of the question may be less likely; it is unlikely the respondents would avoid answering the whole survey because of a relatively small portion of the survey The 2019 Program Director CERA omnibus survey covered 5 main topics including: simulation based medical education, vasectomy training, oncology curriculum, and HIV and Hepatitis C prevention in pregnancy. Because of the nature of the CERA survey, we are unable to compare the cohorts from year to year. There are no data on nonresponders that would allow us to know the impact this issue had on survey response. The CERA survey does not gather data on survey nonresponders; that would be useful information for an additional study. We did not assess all types of pharmaceutical influence including faculty involvement in speakers’ bureaus or industry-funded Continuing Medical Education (CME), faculty participation in ghostwritten or ghostmanaged articles, or involvement in industry-sponsored research. Our survey may miss subtle interactions that influence behavior and resident learning. The effect of an exemption being added in 2013 for interactions with device reps involved in implantable contraceptives is unclear. Tactics used by device manufacturers to market devices largely mirror tactics used by drug companies to market drugs.47⇓–49 Asking participants not to include involvement in implanted device training may have introduced an element of uncertainty for respondents.
In conclusion, pharmaceutical industry influence continues to decrease in US family medicine residencies. National policies and concerns about industry influence on physician behavior continue to transform the nature of the relationship between industry and resident trainees. Concurrently, formal curricula on industry interaction is waning. Programs may be more focused on eliminating the hidden curriculum and discouraging industry interaction than they are on formal curricular change. Residencies will continue to grapple with how best to eliminate or manage influence that leads to nonrational prescribing in a constantly changing health care and educational environment.
Acknowledgments
The authors thank Lorraine Wallace, PhD, CERA advisor; and Richard Gerkin, MD, for statistical support. The authors also thank the co-authors of the 2008 and 2013 survey publications: David Evans, MD; Rachel Trippett, MD; Alicia Bell, MD; Paige Hatcher Dodson, MD; Anthony Fleg, MD; and Jay Siwek, MD.
Notes
This article was externally peer reviewed.
Conflict of interest: None.
Funding: None.
To see this article online, please go to: http://jabfm.org/content/34/1/105.full.
- Received for publication June 11, 2020.
- Revision received August 23, 2020.
- Accepted for publication August 24, 2020.