Abstract
Introduction: Current guidelines classify urinary tract infections (UTIs) in males as complicated and recommend longer treatment than for UTIs in females. The objective of this study is to demonstrate that males with UTIs may be successfully treated with an outpatient 5-day course of levofloxacin.
Methods: Data were obtained from a previously conducted clinical trial (www.clinicaltrials.gov identifier NCT00210886), a multicenter, double-blind, randomized, noninferiority study comparing levofloxacin 750 mg intravenously/by mouth once daily for 5 days and ciprofloxacin 400/500 mg intravenously/by mouth twice daily for 10 days in complicated UTI (cUTI). The current study was a post hoc, subgroup analysis of male and female subjects with cUTI. Subjects were stratified into groups based on sex and antibiotic received. The subjects were analyzed at the end of therapy (EOT) and post therapy (PT) for clinical success rates, defined as no further need for antimicrobial treatment.
Results: Totals of 427 patients (224 male, 203 female) and 350 patients (189 male, 161 female) were included in the modified intent-to-treat (mITT) population and microbiologically evaluable (ME) populations, respectively. Clinical success rates between males and females were not statistically different between antibiotic groups in either the mITT or ME populations at EOT or PT.
Conclusion: This study demonstrates that males with UTI may be treated with a shorter course of antimicrobial therapy for UTI than previously recommended.
- Antibacterial Agents
- Anti-infective Agents
- Ciprofloxacin
- Double-Blind Method
- Levofloxacin
- Outpatients
- Urinary Tract Infections
Infections of the urinary tract caused by bacteria are a common occurrence in clinical practice. In men, however, the length of the urethra, a drier environment surrounding the meatus, and the antibacterial properties of prostatic fluid all contribute to lower rates of urinary tract infections (UTIs).1 Therefore, when UTIs occur in men, they are deemed complicated and subject to longer treatment durations compared with infections in women.1,2 Clinical trials demonstrating the efficacy of antimicrobials in UTIs have typically enrolled a majority of female patients. In general, clinical trials of patients with UTIs enroll small numbers of male patients, and their data are grouped with those of female patients in analyses.3 Drekonja and colleagues4 conducted a retrospective study of >30,000 males with a UTI in the Veterans Affairs system. The objective of their study was to evaluate the recurrence of infection in patients who received a short (<7 days) or long (>7 days) duration of treatment. The investigators found that treatment for >7 days in males with a UTI was not associated with a reduction in early or late recurrence of UTI, but it did increase the risk of Clostridium difficile infection compared with a treatment duration <7 days. The optimal duration of therapy for the treatment of UTIs in males is still unclear, and as a result, recommendations are predominantly based on data extrapolated from clinical trials of essentially all females and from expert opinion.1
The most common pathogen associated with UTIs is Escherichia coli.1⇓–3 Given their broad spectrum of activity and their historic efficacy in treating UTIs caused by E coli, fluoroquinolones are among the first-line antimicrobials to treat these infections.1,2 Unfortunately, widespread use of these agents has led to increased resistance of E coli, and their empiric use is falling out of favor among many clinicians.5⇓⇓–8 A study of patients treated in an emergency department demonstrated that while resistance of E coli to levofloxacin was high (17%), it was significantly greater in those with hospital-acquired UTIs (38% vs 10% of patients with community-acquired UTIs), long-term medical conditions, and fluoroquinolone use within the previous 1 to 4 weeks.7
While resistance to levofloxacin is on the rise, so too is resistance to other antimicrobial agents. The North American Urinary Tract Infection Collaborative Alliance (NAUTICA) trial evaluated 1990 outpatient urinary tract isolates to determine changes in the epidemiology of urinary tract pathogens.9 In that study, 20.5% of isolates came from male patients. Levofloxacin resistance was highest among males aged ≥65 years, at 19.9%. Interestingly, among males in this same age group, resistance to nitrofurantoin and trimethoprim-sulfamethoxazole were higher: 27.8% and 32.6%, respectively.
Peterson and colleagues10 conducted a multicenter, double-blind, randomized, noninferiority study of primarily outpatient subjects comparing levofloxacin 750 mg intravenously (IV) by mouth (PO) daily for 5 days with ciprofloxacin 400 mg IV/500 mg PO twice daily for 10 days in both male and female patients with complicated UTIs (cUTIs) and acute pyelonephritis. In that study, susceptibility of E coli to levofloxacin was very high (94%). Furthermore, the clinical success rates for the short course of levofloxacin were shown to be noninferior to ciprofloxacin. Unfortunately, the published results of that study combined data from both male and female subjects in the analysis. Through reanalysis using the original, unpublished data, the objective of the present study is to evaluate clinical success rates in males with cUTI who received 5 days of levofloxacin or 10 days of ciprofloxacin compared with their female counterparts.
Methods
Through the Yale University Open Data Access Project, deidentified, patient-level data were obtained from a previously conducted clinical trial (www.clinicaltrials.gov identifier NCT00210886), a multicenter, double-blind, randomized, noninferiority study comparing levofloxacin 750 mg once daily for 5 days and ciprofloxacin 400 mg IV/500 mg PO twice daily for 10 days in cUTI and acute pyelonephritis.10 This study was a post hoc, subgroup analysis of male and female subjects with cUTIs only.
Male and female patients at least 18 years old, institutionalized or ambulatory, and diagnosed with a cUTI were included in this study. To be diagnosed with a cUTI, patients had to be male with or without additional complicating factors, or female with at least 1 of the complicating factors listed in Table 1, per the original study protocol.10 Additional inclusion and exclusion criteria can be found in Table 2. Patients were randomized (1:1) to receive either levofloxacin for 5 days or ciprofloxacin for 10 days. Patients randomized to receive levofloxacin were given a placebo in the evening on days 1 to 5 and a placebo twice daily on days 6 to 10. A urine culture was obtained before administration of the first dose of study medication.
Complicating Factors per Study Protocol
Inclusion and Exclusion Criteria
Per the original study protocol, patients were stratified into a modified intent-to-treat population (mITT) if they were randomized to and received at least 1 dose of a study medication, if they were diagnosed with cUTI, and if their culture showed 1 or 2 uropathogens.10 Patients were further classified as microbiologically evaluable (ME) if they followed the study protocol and were not lost to follow-up.
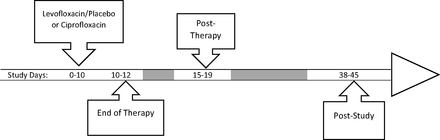
Patients were evaluated at the end of therapy (EOT), post-therapy (PT), and post-study (PS) to determine clinical success rates10 (Figure 1). Patients were deemed to achieve clinical success if they were assessed as “clinically cured” (no signs or symptoms of infection and no need for further antimicrobial therapy) or “clinically improved” (signs or symptoms of infection did not completely resolve but no need for further antimicrobial therapy) by the investigator. Patients were defined as a “clinical failure” if any of the following occurred: no response to therapy, worsening or reappearing signs or symptoms of infection, or additional antimicrobial therapy needed.10
Study timeline.
Statistics
Data from the original trial were obtained from the Yale University Open Data Access Project and analyzed in this study using χ2 and Fisher exact tests, with contingency tables for the categorical data of clinical success rates. Statistical significance was established using a 2-tailed α level of 0.05 (SYSTAT 13 version 13.1). Greater than 90% power was achieved in both the mITT and ME groups (males and females) based on the 15% noninferiority margin used by the original study investigators (PASS 14 software; NCSS, Kaysville, UT).10
Results
A total of 427 patients (224 male and 203 female patients) with cUTI met all inclusion criteria and were analyzed in the mITT group. Of those 427 patients, 350 (189 male, 161 female) adhered to the study protocol and were included in the ME group (Figure 2). Baseline demographics of the patients are listed in Table 3; approximately half of the patients in the mITT population were males and the majority of subjects were white, over the age of 60, and infected with E coli. Nearly all were treated in an outpatient setting, rather than in a hospital.
Patient disposition. cUTI, complicated urinary tract infection; mITT, modified intent-to-treat; ME, microbiologically evaluable.
Baseline Demographics of the Modified Intent-to-Treat Population
Overall, the clinical success rates for males and females with cUTI were similar in both the mITT and ME populations at EOT and PT (Table 4). In the mITT population, males who received levofloxacin had a success rate of 83% at EOT and 76% PT, compared with their female counterparts, who experienced success rates of 81% both EOT and PT (P = .73 and .411, respectively). In the ME population, the clinical success rate for males who received levofloxacin at EOT and PT were 87% and 81% compared with 94% and 86% in females, respectively (P = .141 and .428, respectively). Similarly, there were no statistical differences between males and females who received ciprofloxacin in the mITT and ME populations at both EOT and PT. Furthermore, when comparing the clinical success rates of either a 5-day course of levofloxacin or a 10-day course of ciprofloxacin in the male population alone, no significant differences were found in the mITT or ME population at EOT or PT (Table 5).
Comparison of Clinical Success Rates between Male and Female Patients with Complicated Urinary Tract Infection
Clinical Success Rates for Male Patients with Complicated Urinary Tract Infection
Further analyses were performed within the male population to determine clinical success rates for subjects with additional complicating factors (Table 6). Patients who received levofloxacin had a statistically similar rate of clinical success as those who received ciprofloxacin for all additional complicating factors, with the exception of those with a catheter. In this cohort of patients, the clinical success rate with levofloxacin (91.3%) was significantly higher than those who received ciprofloxacin (56.3%; P = .019).
Comparison of Clinical Success Rates within Complicating Factor Groups in the Microbiologically Evaluable Male Population at the End of Therapy, Based on Antibiotic Received
Comparison of the clinical success rates among male patients within the antimicrobial group (ciprofloxacin or levofloxacin), based on additional complicating factors, yielded significantly different results (Table 7). In this analysis, males who received ciprofloxacin were less likely to have clinical success if they had a catheter, neurogenic bladder or urinary retention, and ≥2 complicating factors, compared with patients who received ciprofloxacin without the associated complicating factor (56.3% vs 91.5%, P = .002; 69.2% vs 91.7%, P = .009; and 70.4% vs 92.3%, P = .04, respectively). By contrast, there was no significant difference in clinical success rates among males with these complicating factors who received levofloxacin.
Comparison of Clinical Success Rates within Antibiotic Groups in the Microbiologically Evaluable Male Population at the End of Therapy, Based on Additional Complicating Factors
Discussion
The results of this subgroup analysis suggest that male patients with UTIs, with or without additional risk factors for cUTIs, achieved clinical success with a 5-day course of levofloxacin, rather than a traditional, prolonged course (7–14 days) of therapy. Furthermore, male patients in this analysis had clinical success similar to that of their female counterparts in both the 5-day course of levofloxacin and the 10-day course of ciprofloxacin. In addition, the results suggest that male patients with complicating risk factors have a higher rate of clinical success with a 5-day course of levofloxacin than a 10-day course of ciprofloxacin. While it would be unwise to conclude that high-dose, short-course levofloxacin should be used in all male patients with cUTIs, the data presented here support its utility for outpatient therapy.
Even though the results of this analysis are positive and suggest shorter courses of antibiotics can be considered in males with UTIs, there are limitations to this study. One caution when applying these results to the male population is to not deviate from the original inclusion and exclusion criteria, as well as the added exclusion criteria for our analysis—acute pyelonephritis. While the original study did not exclude patients with acute pyelonephritis, there were only 18 males with the diagnosis. As such, to represent a fair comparison of patients, the data from all patients with acute pyelonephritis were excluded from this analysis. Because of this, it would be inappropriate to apply these results to males with acute pyelonephritis. In addition, male patients with prostatitis, epididymitis, or perirenal abscesses were excluded from the study and should be treated according to currently recommended treatment algorithms rather than 5 days of levofloxacin. Furthermore, nearly all patients included in the study were outpatients, rather than hospitalized. Therefore it would be difficult to apply these results to hospitalized patients.
The results of this study should be applied only to susceptible microorganisms, in regions where resistance of uropathogens to levofloxacin is relatively low, and in patients with few to no risk factors for E coli resistance.7 As stated previously, the subjects in this study were nearly entirely treated in an outpatient setting where the resistance of E coli to the fluoroquinolones was low. Therefore, attempting to infer clinical success of levofloxacin in a environment where E coli resistance to fluoroquinolones is known to be high might be futile. Several studies have demonstrated increasing resistance of uropathogens to not only levofloxacin but also other agents typically used to treat these infections.7⇓–9 Globally, E coli resistance to fluoroquinolones is becoming significant, ranging from 2% to 69% for those with uncomplicated, community-acquired UTIs and up to 98% for those cUTIs.11 Because of this, it becomes important to treat such infections with the agent that has the greatest likelihood of clinical success. While a fluoroquinolone may not be the first option to treat some UTIs, it would be reasonable to treat outpatient males without previous exposure to fluoroquinolones (within the previous 4 weeks) with a 5-day course of levofloxacin 750 mg PO daily. Close follow-up to ensure resolution of symptoms is also advised, especially when the causative organism is unknown.
While the results were not reported in the previous trial, because of the small samples, in our study there were some interesting findings among those patients who clinically failed therapy. In males who received ciprofloxacin, 12 of the 14 clinical failures (85.7%) occurred with E coli as the pathogen in the ME group PT. By contrast, only 6 of the 17 clinical failures (35%) in the levofloxacin group were infected with E coli. As a result, clinical success rates for male patients initially infected with E coli were 86% and 77% for levofloxacin and ciprofloxacin, respectively.
One analysis not performed in our post hoc study was the evaluation of microbiologic eradication rates (elimination or reduction to <104 colony-forming units/mL of uropathogens at study entry), despite being performed in the original trial. In the male population, urine cultures were not available at EOT for approximately 12% of subjects in the ME group, and therefore evaluation of microbiologic eradication rates was not included as an objective of this study. For male patients in the ME group PT, however, microbiologic eradication rates were found to be 72 of 91 (79.1%) and 84 of 98 (85.7%) in the levofloxacin and ciprofloxacin groups, respectively.
Finally, the US Food and Drug Administration (FDA) recently advised health care professionals and consumers not to use fluoroquinolone antibiotics for uncomplicated infections because of increased risks of serious adverse events that outweigh the benefits of therapy.12 Furthermore, the FDA stated that fluoroquinolones should only be used when alternative agents are not available to treat these types of infections. In the original study, the authors reported 192 patients in the levofloxacin group and 185 patients in the ciprofloxacin group had at least one adverse event; the most common were nausea, headache, and diarrhea.10 The authors went on to state that 17 patients in the levofloxacin group and 15 in the ciprofloxacin group had significant adverse drug events. Unfortunately, they failed to report any episodes of C difficile infection, QTc prolongation, or tendon rupture—all of which can occur with these agents. Furthermore, while adverse events associated with fluoroquinolone use can occur after a single dose, the risks increase with prolonged courses.13 Given this information and the recent advice from the FDA, it is particularly important to give the shortest, most effective duration of fluoroquinolone therapy for the treatment of complicated infections. The results of our analysis suggest that if a fluoroquinolone is used in males with a UTI, short courses may be as effective as longer courses, thereby decreasing their risk of serious adverse events.
While the results of this analysis demonstrated the utility of levofloxacin in males with cUTIs in an environment where resistance was low, an even greater finding was that the clinical success rate of a short course of antibiotics to treat cUTI was similar in male and female patients. Without question, 1 contributing factor leading to increased bacterial resistance is prolonged use of antimicrobial agents; therefore, shorter courses in males with cUTIs could help alleviate some of the resistance problems.14,15 A larger-scale study comparing short courses of antimicrobials in males and females should be conducted to validate the results of this study. Until then, in outpatient males with cUTIs without acute pyelonephritis or other exclusions defined in this study, we recommend a duration of therapy similar to that used for female patients. Furthermore, if this strategy is used, we recommend follow-up with an appropriate health care practitioner to ensure clinical success.
Conclusion
Males who develop a UTI have historically been given the classification of cUTI, and therefore it has been suggested that they be treated for an extended duration. The results of our analysis suggest shorter courses of levofloxacin are as effective as longer courses of ciprofloxacin in males in the outpatient setting. Furthermore, levofloxacin may be more effective than ciprofloxacin when males have additional complicating factors.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
Disclaimer: This study, conducted under Yale University Open Data Access Project no. 2015-0514, used data obtained from the Yale University Open Data Access Project, which has an agreement with Janssen Research & Development, LLC. The interpretation and reporting of research using this data are solely the responsibility of the authors and do not necessarily represent the official views of the Yale University Open Data Access Project or Janssen Research & Development, LLC.
- Received for publication February 11, 2016.
- Revision received May 16, 2016.
- Accepted for publication May 19, 2016.