Abstract
Hepatitis C is a common cause of cirrhosis, hepatocellular carcinoma, and liver transplant. Although it is usually asymptomatic, new screening recommendations will lead to increased recognition by primary care physicians. Rapidly evolving treatment recommendations are making this a treatable infection for many patients. Recognition of the infection and initiation of treatment for appropriate patients will decrease the likelihood of progression to cirrhosis and hepatocellular carcinoma. Primary care physicians have the difficult task of managing comorbid conditions, such as chronic pain and hyperlipidemia, in patients with hepatitis C, as well as a potential for treating hepatitis C.
Hepatitis C is the leading cause of liver transplant in the United States and a common cause of cirrhosis and hepatocellular carcinoma.1,2 An estimated 4.1 million individuals, or 1.6% of the population, are infected nationwide, many of whom are unaware of the infection since it is often asymptomatic.3 Hepatitis C is a bloodborne infection, most commonly transmitted by intravenous drug use.2
Screening for Hepatitis C
Screening for hepatitis C should be considered in patients who are at risk for infection or who have known exposure (Table 1). The Centers for Disease Control and Prevention and the US Prevention Services Task Force recommends one-time screening for people born between 1945 and 1965 because of the high rate of undiagnosed infection in this population who may have been exposed before universal precautions were implemented and do not recall or report risk factors to their primary care providers.4⇓–6 Screening patients with risk factors for hepatitis C has a sensitivity of 90%, with a number needed to screen to identify one case of hepatitis C of <20.7
Clinical Presentation
Jaundice, abdominal pain, or, more commonly, nonspecific flu-like symptoms such as fatigue, muscles aches, and nausea occur in 25% to 30% of patients when they are infected with hepatitis C, but most patients are asymptomatic.8 During this period, alanine aminotransferase and aspartate aminotransferase levels increase rapidly, often to 10 times the upper limit of normal.9
Approximately 50% to 80% of patients infected with hepatitis C progress to chronic infection, defined as a persistence of the virus for >6 months after the initial infection.2 Chronic hepatitis C is usually asymptomatic and often is found during investigation for an incidental finding of elevated liver transaminases. When symptomatic, patients with chronic hepatitis C present with nonspecific symptoms such as nausea, anorexia, and fatigue or signs of cirrhosis on examination.
Hepatitis C has been reported to cause numerous extrahepatic manifestations, although the association for many of the conditions has been questioned.10 Hepatitis C can cause both mixed cryoglobulinemia and B-cell non-Hodgkin's lymphoma11 and may have a role in certain rheumatologic, endocrine, and dermatologic conditions such as rheumatoid arthritis and dermatomyositis.10,11
Diagnosis
In patients with acute hepatitis C, hepatitis C virus (HCV) RNA can be detected as early as 7 days after exposure, but the antibody to HCV (anti-HCV) may not be present until 6 to 8 weeks after exposure.9 Chronic hepatitis C is diagnosed when both anti-HCV and HCV RNA are present (Figure 1).9 Anti-HCV is the appropriate laboratory test to order when screening for hepatitis C in asymptomatic patients; hepatitis C is confirmed by the presence of HCV RNA.6
Initial Evaluation of Patients With Chronic Hepatitis C
To determine whether a patient is a potential candidate for treatment, they must be evaluated for contraindications to treatment and disease severity (Tables 2 and 3). Patients have traditionally required a liver biopsy to determine the severity of liver disease, although the utility of liver biopsy has recently been called into question because it carries a small but real risk of complications.2 Serum tests and ultrasonography may assist in defining disease severity but are not recommended by current guidelines.14
Patients with compensated cirrhosis (bilirubin <1.5 g/dL, international normalized ratio (INR) <1.5, albumin >3.4 g/dL, platelets >75,000 k/mm3, and no evidence of ascites or hepatic encephalopathy) or fibrosis on liver biopsy should be offered treatment for chronic hepatitis C.2 Current treatment guidelines stress that the decision to treat should be individualized based on the patient's overall health and personal preferences. Therefore, while the data suggest that patients without evidence of fibrosis or cirrhosis on biopsy have a low risk for liver-related complications or death in the next 10 to 20 years, some may still elect to pursue treatment.2 Patients who elect not to pursue treatment may be monitored with annual assessment using liver function and consideration of biopsy if aminotransferase levels increase.2
Treatment
Hepatitis C is treated to lower the risk of progression to end-stage liver disease or hepatocellular carcinoma. The goal of treatment is a sustained decrease in hepatitis C viral RNA (called the sustained virological response [SVR]), which is defined as no detectable hepatitis C viral RNA 24 weeks after treatment.2 Studies have found that SVR is linked with a 30% to 50% decrease in all-cause mortality.15
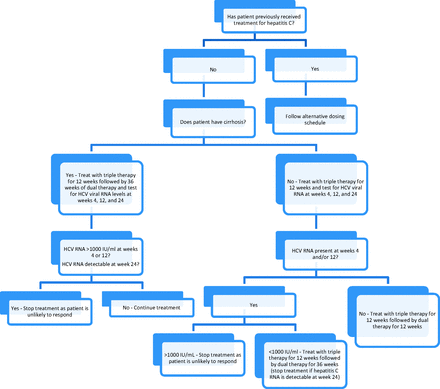
The treatment regimen depends on the genotype of the virus. For genotype 1, the most common type in the United States, patients are treated with triple therapy, which includes pegylated interferon-α, ribavirin, and either boceprevir and telaprevir, which are direct-acting protease inhibitors.2,16,17 The addition of the direct-acting protease inhibitors significantly increases the likelihood of successful treatment as determined by SVR in patients infected with genotype 1 and shortens the duration of treatment for some patients.16 While the 2 agents have equal efficacy, telaprevir has a simpler dosing schedule and for that reason is discussed in detail here (Table 4 and Figure 2). Patients with genotypes 2 to 6 are treated with pegylated interferon and ribavirin at a dosage of 800 mg daily for 24 weeks.17
Treatment of chronic hepatitis C genotype 1 with telaprevir-based therapy. Dual therapy, peginterferon + ribavirin; HCV, hepatitis C virus; RNA, ribonucleic acid; triple therapy, telaprevir + peginterferon + ribavirin (see Table 4 for dosing).
Both protease inhibitors have multiple drug interactions and should be checked against all of a patient's current medications.16 Anemia is a frequent complication of treatment, occurring in as many as 49% of patients treated with protease inhibitors, and it may be treated by reducing the dose of ribavirin.16 Other common side effects of treatment include rash, pruritus, nausea, and diarrhea.16
The treatment of hepatitis C is rapidly evolving, with >40 drugs in development, including interferon and ribavirin analogs, vaccines, immunomodulators, and direct-acting antiviral drugs.18 As the treatment of hepatitis C continues to evolve, primary care physicians may also have a more active role in the treatment of hepatitis C, and some locations are already doing so with the assistance of telemedicine.19,20
Primary Care for Patients with Chronic Hepatitis C
Patients who are not immune to hepatitis A or B should receive vaccination.21,22 Patients with cirrhosis should also be offered pneumococcal vaccination and annual inactivated influenza vaccination.23
All patients with chronic hepatitis C should be advised to abstain from drinking alcohol to decrease the risk of cirrhosis, and referral to treatment facilities should be provided.24 Alcohol significantly increases the risk of the development of cirrhosis in patients with hepatitis C, with an odds ratio of 147.2 (95% confidence interval [CI], 42.1–514.3) for heavy drinkers (eg, >175 g per day averaged over the lifetime).25 Even moderate drinking can increase the risk for progression to cirrhosis in some patients.26 Intravenous drug abusers should be referred for appropriate addiction treatment. Obesity is a risk factor for progression of liver disease because of the development of nonalcoholic steatohepatitis syndrome, so assistance with weight loss should be provided to obese patients.27
Patients should be counseled about behaviors to reduce the risk of transmission, including avoiding the donation of blood and plasma. The risk of transmission of hepatitis C by sexual activity in monogamous heterosexual couples is low (approximately 0.07% per year; 95% CI, 0.01–0.13%), and condom use is not necessary in these couples.28 Sexual transmission may occur at a higher rate in men who have sex with men, especially those infected with HIV.29 Patients should be counseled to avoid sharing toothbrushes, razors, and other items that may be contaminated with blood.
Chronic hepatitis C infection should not be considered a contraindication to treatment with HMG-coenzyme A reductase inhibitors (statins)30 and may even be an option for the treatment of hepatitis C in the future.31,32 Statins should be avoided if there is evidence of hepatic failure, such as jaundice or increased bilirubin level.33 Patients with elevated serum aminotransferase levels (>3times baseline or >5 times the upper limits of normal) should not be taking statins, although they could be started if levels fall.34
Treatment of pain in patients with cirrhosis can be particularly problematic. Many providers avoid using acetaminophen in patients with chronic liver disease because of the potential for hepatotoxicity. However, at therapeutic doses of <4 g/day, acetaminophen is generally considered safe, although some experts recommend a lower dose of 2 g/day.35,36 Nonsteroidal anti-inflammatories should be avoided in patients with cirrhosis because of the potential for hepatorenal syndrome or gastrointestinal bleeding.37,38 Because of impairment in drug metabolism, the use of opioids should be minimized since they can precipitate hepatic encephalopathy. If opioids are necessary for adequate analgesia, the minimum effective dose should be used and longer intervals between administrations should be considered.39
Primary care physicians may also be asked to manage depression induced by treatment with interferon-α. In general, treatment with selective serotonin reuptake inhibitors can be initiated as soon as patients begin to manifest symptoms of depression.40 Several small trials have investigated the use of selective serotonin reuptake inhibitors for the prevention of depression, with mixed results.41 Therefore, current data support waiting for symptoms to develop rather than using pretreatment.
Patients who have both hepatitis C and cirrhosis are at increased risk of developing hepatocellular carcinoma and should be offered screening by annual or semiannual ultrasound. α-Fetoprotein levels alone should not be used for screening unless ultrasound is unavailable.42 Current guidelines also recommend continued screening for patients who have been successfully treated for hepatitis C and patients who have evidence of bridging fibrosis on liver biopsy, although the data of efficacy lag behind the recommendation.42
Conclusion
With emerging recommendations to screen for asymptomatic hepatitis C in patients born between 1945 and 1965, family physicians will increasingly be responsible for the diagnosis and management of infected patients. Ordering appropriate laboratory work and maximizing control of chronic conditions before initiating referrals can help ensure selected patients receive prompt treatment. Primary care providers can work with gastroenterologists and infectious disease specialists to ensure patients receive appropriate vaccinations, lifestyle counseling, and screening tests (Table 5).
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
- Received for publication May 23, 2013.
- Revision received August 31, 2013.
- Accepted for publication September 6, 2013.