Abstract
Objective: Although many coronary heart disease (CHD) risk factors are known, the role of an individual's changing personal health history is unclear. We implemented this study to evaluate whether accounting for previous Framingham Risk Scores (FRSs) improves the predictive ability of a current FRS for future CHD in middle-aged adults.
Methods: We analyzed data from the Atherosclerosis Risk in Communities Study (ARIC), a longitudinal cohort of people 45 to 64 years old at entry (1986 to 1989 through 2001). FRSs were calculated for participants in the ARIC cohort (3901 men, 5406 women) at baseline (visit 3) and 3 and 6 years before. Using Cox regressions we evaluated the risk of CHD development for the FRS 6 years from baseline and then evaluated whether the addition of the change in FRS assessments from 3 and 6 years before the baseline improved the predictive ability of the FRS. Areas under the receiver operating characteristic (AUROC) curves were compared.
Results: The addition of the difference between the baseline FRS (eg, in 1995) and the FRS from 6 years earlier (eg, in 1989) to predict CHD development by 2001 for the entire cohort yielded an AUROC of 0.730, which was a significant improvement over just using the baseline FRS (P < .05). The effect was located primarily among women, with the AUROC curve improving from 0.667 to 0.709 (P < .05). There was no improvement for CHD risk prediction in men when the earlier FRS assessments were taken into account. Men seem to have less change in some risk factors over time.
Conclusions: Accounting for an individual's history improves risk assessments based on current measures.
Coronary heart disease (CHD) is a leading cause of morbidity and mortality.1,2 A strategy for the primary prevention of CHD is an identification of asymptomatic individuals at higher risk and the implementation of potential interventions to reduce their future risk. This is consistent with current clinical practice guidelines, including Adult Treatment Panel-III, Joint National Committee-VII, and American Heart Association recommendations.3–5
Because there are many risk factors for CHD, various studies have attempted to move beyond simply looking at one risk factor at a time but rather to improve prediction using multiple variables.6–8 These multivariable risk scores are primarily based on the general strategy of assessing conventional risk factors like blood pressure, smoking, diabetes, and lipids.6,7,9 Because nearly 20% of people who develop CHD do not have one of the primary conventional risk factors, other markers have been investigated as ways to improve CHD risk prediction.10
In particular, the addition of novel biomarkers like C-reactive protein and homocysteine to models composed of conventional risk factors has been evaluated.11–14 Unfortunately, except for the rare example of C-reactive protein, which has yielded conflicting results, the addition of novel biomarkers has not improved the prediction of models based on conventional risk factors. A potential reason for the lack of benefit in adding novel biomarkers to conventional risk factors or even a point of improvement in conventional risk factors themselves may be that these strategies tend to be based on current assessments of risk factors that may be transient, thereby missing the cumulative impact of oxidative stress, dyslipidemia, and inflammation.
There is some evidence that personal history of risk factors may influence the validity of current risk factor assessments. For example, people who stop smoking cigarettes can bring their risk of cardiovascular disease back to that of people who have never smoked.15 However, this process may take several years. In fact, for heavier smokers, a return to the risk similar to someone who has never smoked may require at least 10 years.15–17 Thus, if a measure like the Framingham Risk Score (FRS) simply includes an evaluation of current smoking status, the historical impact of previous smoking would not be measured even though it may influence the current assessment of cardiovascular disease or CHD risk.
Primary care physicians many times have ongoing and continuous relationships with their patients. Consequently, they have the ability to measure the patient's risk factors at multiple times. Current risk assessment strategies assume independence of each of these multivariable scores, when in fact each assessment in a single person are not independent. It is possible that the actual CHD risk could be improved by accounting for the previous risk factor assessments. The purpose of this study was to evaluate whether accounting for previous FRSs improves the predictive ability of the FRS for future CHD in middle-aged adults.
Methods
We conducted an assessment of the public-use data set of the Atherosclerosis Risk in Communities (ARIC) Study. The ARIC is a cohort of 15,792 participants from 4 US communities aged 45 to 64 years. The locations include Forsyth County, NC; Jackson, MS (African-Americans only); the suburbs of Minneapolis, MN; and Washington County, MD. In the public-use data set, participants are classified as either nonblack or black. The nonblack participants are mostly white, but include 14 American Indians and 34 Asian participants. The ARIC baseline examination (visit 1) was during the period of 1986 to 1989, and follow-up examinations were during 1990 to 1992 (visit 2), 1993 to 1995 (visit 3), and 1996 to 1998 (visit 4). The cohort was followed with annual contact through December 31, 2001. We conducted a longitudinal analysis using information from visits 1, 2, and 3 to classify participants and then followed them from visit 3 to 6 years later for the development of incident CHD (Figure 1). People with diabetes and a diagnosis with CHD before visit 3 were excluded because the FRS was not applicable to them.
Twelve-year study timeline.
Coronary Heart Disease
The outcome is incident CHD in 6 years. CHD was defined as a myocardial infarction, fatal CHD, or cardiac procedure. ARIC participants had follow-up interviews annually, usually within a month of their anniversary date for enrollment. Follow-up interviews included questions based on the Rose questionnaire for CHD.18 If the participant indicated a hospitalization, ARIC abstractors reviewed and recorded discharge diagnoses. Community surveillance involved obituary reviews as well as community death certificate surveillance for mortality. If mortality was identified, there was similar record abstraction for in-hospital deaths or an interview with the family if death occurred outside of the hospital. ARIC participants gave previous consent for hospital record review and interviews with family members in the event of death. A special ARIC Morbidity and Mortality Classification Committee reviewed all hospitalizations and deaths to review and assign diagnoses for cardiovascular events and causes of death based on defined criteria.
Framingham Risk Score
Although the FRS for CHD has been computed in a variety of different ways since it was first presented, we used the version presented in the Adult Treatment Panel III.3 We computed the FRS at visit 1, visit 2, and visit 3. The FRS can be used for individuals without previous CHD or diabetes and is computed using age, sex, measured total cholesterol, measured high-density lipoprotein cholesterol, systolic blood pressure, current medication for hypertension, and current smoking status.
Analysis
First, we used the FRS at visit 3 to compute 6-year CHD risk to represent the standard strategy of using a multivariable risk score based on current assessment of risk factors. We did this for the entire sample, using sex as a covariate while acknowledging that the FRS is scored differently for men and women. Second, we used several strategies to evaluate the addition of the patient's personal history of risk factors to the current FRS. As shown in Figure 1, we calculated the FRS at visit 1, visit 2, and visit 3, representing 6 years before baseline, 3 years before baseline, and baseline, respectively. We computed (1) the difference in risk points between the FRS at 3 years before (visit 2) and at baseline (visit 3) and (2) the difference in risk points between the FRS at 6 years before (visit 1) and at baseline (visit 3). We computed Pearson's correlations between the risk scores at each time point.
To obtain parameter estimates, we then conducted Cox regressions by sex separately to predict incident coronary heart disease over 6 years. The regressions were computed separately by sex because that is consistent with the FRS scoring. The Cox regressions were (1) the FRS at baseline, (2) the FRS at baseline plus the difference in risk points between 3 years before to the baseline, and (3) the FRS at baseline plus the difference in risk points between 6 years before and baseline. For Cox regression analyses we used the statistical package SAS version 9.1 (SAS Institute Inc., Cary, NC). The proportionality of the Cox regression hazard ratios were compared by Schoenfeld residuals. The variables in each model were multiplied with their respective parameter estimates and these values summed to produce scores for each participant in each model. Receiver operating characteristic (ROC) curves were then computed for each model using MedCalc and the areas under the curve compared.
Time to CHD events was used in the Cox's regressions, but not in the ROC curve analyses. For the ROC curve analyses, CHD events any time within 6 years of baseline were classified as belonging to the positive group and participants without CHD events classified as belonging to the negative group.
Results
The characteristics of the sample at baseline (visit 3) are shown in Table 1. The number of individuals progressing to a CHD event over 6 years was 299 men and 131 women.
Characteristics of the Cohort at Baseline
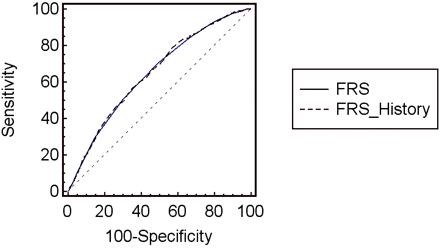
Results of our ROC curve analyses are shown in Table 2 and Figures 2 and 3. The Schoenfeld analyses indicated that the hazards ratios from the Cox regressions were proportional. In the standard model estimating from the visit 3 assessment, the 6-year prediction of the FRS yielded areas under the ROC curve of 0.720 for entire sample, 0.646 for men, and 0.667 for women. When we added the difference between the FRS at visit 3 and an FRS from 3 years before, the areas under the ROC curve increased but were not significantly different from the FRS at baseline alone. However, when the difference between the FRS at baseline (visit 3) and 6 years before was added to the baseline FRS assessments on each individual, this new strategy offered significantly improved prediction for the development of CHD for the entire population and among women. This finding was not yielded among men.
Receiver operating characteristic curves for Framingham Risk Score (FRS) at baseline and FRS at baseline plus the difference in risk points between 6 years previous and baseline (FRS History) among men.
Receiver operating characteristic curves for Framingham Risk Score (FRS) at baseline and FRS at baseline plus the difference in risk points between 6 years previous and baseline (FRS History) among women.
Areas Under the Receiver Operating Characteristic Curves for Models Predicting 6-Year Coronary Heart Disease Risk Incorporating Changes in Risk Scores Over Time
The correlations between the FRS at the baseline and previous assessments tend to be relatively similar between men and women (Table 3). However, the correlation between baseline and 6 years earlier is higher among men than among women. There is less stability among women, particularly in relation to current smoking status. A substantial decline in current smoking among women (9%) who had a CHD event suggested a carryover effect of previous smoking on current risk (Table 4).
Correlations Between Framingham Risk Score Risk Points at Different Times
Proportion of Current Smokers by Sex, Coronary Heart Disease Event Status Over 6 Years of Follow-up and Time (at Baseline or Before Baseline)
Discussion
The results of this study indicate the value of the addition of an individual's personal history of CHD risk factors to current state assessments when predicting future CHD risk. This finding provides a potential explanation for the lack of additional value of many current state biomarkers with conventional risk factors; it points to the need for a measure that assesses a cumulative risk. The value of using historical information in CHD risk assessment was evident in women and not supported in men.
It is unclear why in this particular analysis history had an added benefit to the FRS for women but did not add significantly to the predictive power of the FRS in men. Analyses of individual components of participants’ history showed that no single component improved the model for women. The benefit of adding the FRS from 6 years before seemed to be because of changes in multiple components of FRS history. Consistent with this sex difference is the recent finding that men are less likely to change/adopt healthy habits in middle age; thus their FRS would tend to remain stable and decrease the value of history as a factor.19 It may be useful for primary care physicians to be particularly attuned to the possibility of changes in risk factors among their female patients.
It is not surprising that men and women may differ in the impact of past risk factors and future CHD risk. Several studies have examined women separately, whereas others have shown that men and women differ in the association between major risk factors and myocardial infarction.20,21 For example, in one cohort of more than 2000 adults aged 45 to 64 years at baseline, the results showed that the risk of CHD for people with diabetes in analyses adjusting for conventional risk factors was 2.8 for men and 9.5 for women.22
Because of the past performance of inherited characteristics like family history in CHD, some studies have attempted to use genes as risk markers for CHD.7,23,24 The attempts to use genes as predictors of risk for CHD have been less than satisfactory. In fact, in a study using the ARIC cohort, adding a genetic risk score comprising multiple genetic variants to a conventional risk factor score did not significantly increase in predictive ability the model above that provided by current conventional risk factors for white adults.7 This suggests that the phenotypic expression of genes associated with CHD may already be expressed in risk factors for CHD like hypertension and hyperlipidemia. However, the current findings suggest that an individual's history may be more useful than adding genes to conventional risk factors in improving CHD risk prediction models.
One study that considered an indicator of cumulative damage as an adjunct to the FRS focused on the addition of coronary artery calcium score.25 The coronary artery calcium score represents subclinical atherosclerosis and is predictive of total plaque burden. The addition of coronary artery calcium score improved the prediction of the FRS, particularly among patients with intermediate FRS risk. The present results of the addition of previous FRS measurements to the current measure support the coronary artery calcium score findings regarding assessments representative of cumulative damage. However, the ability to do multiple FRS measures is more easily undertaken in primary care than are computed tomography scans for coronary artery calcium score. Moreover, as more primary care offices adopt electronic health records the more historical information that will be able to be used by the clinician, thereby allowing for better assessments of risk.
Limitations of the study include generalizabilty from a study population of only 4 communities, although the ARIC population is diverse and geographically dispersed across the United States. Another limitation is that, although the cohort allowed us to evaluate the FRS at 3 different time points and follow patients forward to the development of CHD, the predictive models may be improved by adding more history and having a 10-year rather than 6-year follow-up.
Conclusion
This study provides support for the need to use a cumulative history measure of risk in CHD prediction. The practical significance of these findings is that a patient's history of risk factors does matter in future CHD risk even if those risk factors no longer exist. Future studies focusing on ways to incorporate a cumulative variable of a person's risk factor history may hold particular promise for risk prediction. Because of their continuous relationships with patients, primary care physicians are well-positioned to measure the FRS at multiple time points and thereby provide patients with an improved picture of their CHD risk.
Notes
This article was externally peer reviewed.
Funding: The Atherosclerosis Risk in Communities Study (ARIC) is conducted and supported by the NHLBI in collaboration with the ARIC Study Investigators. This manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the ARIC Study or the NHLBI. This project was supported in part by grant 1D14 HP 00161 from the Health Resources and Services Administration, grant 1 P30AG021677 from the National Institute on Aging, Grant R01 HL076271 from the National Heart Lung and Blood Institute, and a grant from the Robert Wood Johnson Foundation
Conflict of interest: none declared.
- Received for publication February 20, 2008.
- Revision received March 31, 2008.
- Accepted for publication April 11, 2008.