Abstract
Background: Obese people have a higher prevalence of cardiovascular disease, but the mechanism of this result remains obscure. The purpose of this study was to prove heart rate variability (HRV) response at rest and during stimuli in these persons.
Methods: The subjects were 41 healthy persons (19 men, 22 women) ranging in age from 20 to 65 years. HRV was measured at rest and at given stresses with noise and standing.
Results: Higher levels of fat mass, percentage fat content, and waist/hip ratio were significantly associated with lower low frequency (LF) (r = −0.34, r = −0.43; P < .01, r = −0.33, P < .05), and lower root mean square differences of successive NN intervals (RMS standard deviation) (r = −0.33, r = −0.35, r = −0.38, P < .05). During rest, noise, and standing, the change amount of the standard deviation of NN interval (SDNN) and low frequency/high frequency ratio were not different between normal and obese groups (P > .05).
Conclusion: Although there was no significant HRV response to stimuli, root mean square of successive differences (which reflects parasympathetic acivity) and low frequency (which mainly reflects sympathetic activity) were negatively correlated with fat mass, fat percentage, and waist-to-hip ratio at rest in obese persons. These results mean obesity can change cardiac autonomic nervous response, meaning that the mechanism by which obesity increases cardiac mortality would be explained, at least partially.
In Korea, as in Western countries, disease related to obesity has increased recently. However, body mass index (BMI) of patients who have obesity-related diseases is much lower in Korea compared with similar patients in Western countries. For example, average BMI in patients with non-insulin-dependent diabetes mellitus is 24.2 ± 3.4 kg/m2. The percentage of overweight patients with insulin-dependent diabetes mellitus whose BMI is higher than 25 kg/m2 is 36%.1,2 This is very low compared with the US level of 60% to 69%. In addition, some studies showed that the number of insulin-resistant patients with a BMI above 23 kg/m2 sharply increased.3,4 This is because of the higher susceptibility of Koreans to diseases related to sugar and fat because of their smaller bodies compared with persons from Western countries.2,5,6 Therefore, in Korea, a BMI of 25 kg/m2 is considered the cutoff value for obesity and 23 kg/m2 is considered the cutoff value for overweight.7,8 These criteria are compatible with World Health Organization Asian9 and Japanese10,11 obesity criteria.
Despite the relatively consistent findings of increased prevalence of cardiovascular disease in obesity, the reason for these associations remains obscure. Many factors have been suggested as causes for this relationship, such as insulin resistance, hypertension, and reduced high-density lipoprotein. On the other hand, it has also been suggested that a reduction in autonomic function might be the mechanism for the increased prevalence of cardiovascular disease in obesity.12–18 However, these studies have not concentrated on the autonomic activity of the heart itself. Heart rate variability (HRV) measures the effect of autonomic function on the heart alone. Therefore, it could be the most useful method by which to investigate the effect of obesity on cardiovascular disease. It is important to emphasize the effect of obesity on HRV; decreased HRV significantly increases cardiovascular mortality.19–23 Because obesity is related to increased morbidity and mortality in cardiovascular diseases.24–26
HRV represents continuous fluctuations in heart rate. R-to-R interval variations on electrocardiograms represent beat-to-beat control mechanisms. Efferent sympathetic and parasympathetic activities directed to the sinus node characterized by each cardiac cycle can be modulated by central and peripheral stimulators. These stimulations generate rhythmic fluctuations in efferent neural discharge that manifest as oscillations in the heart beat period.27,28
HRV can be measured by 2 methods: the time domain method and the frequency domain method. In the time domain method, the standard deviation of NN interval (SDNN) and the root mean square of successive differences in NN intervals (RMSSD) are obtained. SDNN reflects all the cyclic components responsible for variability in the periods of recording, and RMSSD mainly reflects parasympathetic nervous activity. Proper mathematical algorithms can be used to obtain the frequency. In the frequency domain method, the high frequency (HF; 0.15 to 0.40 Hz) component is associated solely with parasympathetic activity.29–31 The low frequency (LF; 0.04 to 0.15 Hz) component is associated with both sympathetic and parasympathetic activity, but sympathetic activity is the greater contributor.30,32–34
This study was performed on the assumption that obese persons have a different HRV response at rest and to given stimuli. If obesity can change the HRV response to stimuli as well as the rest state, meaning that obesity can change cardiac autonomic nervous response, so the mechanism by which obesity increases cardiac mortality would be explained, at least partially. To investigate this hypothesis, HRV was measured at rest and in response to such stimuli as noise and standing.
Methods
Study Subjects
The subjects were 41 healthy persons (19 men and 22 women) ranging in age from 20 to 65 years. They had had a general health check up less than 12 months before the test. All had normal fasting blood glucose (<126 mg/dL) and were normotensive (<140/90 mm Hg). None of the subjects was taking medication that might affect the autonomic nervous system. Results of liver, renal, and endocrine function tests were all normal.
An institutional review committee approved the experimental protocols of this study, and all subjects gave informed written consent for participation. Participants in the study abstained from tobacco, caffeinated beverages, and alcohol on the morning of the test day. The tests were performed between 11:30 am and 1:30 pm and at least 3 hours after a meal.
Experiments
Obesity Index Measure
The height (cm) and weight (kg) of each participant were measured at the beginning of the study. BMI was calculated as the weight in kilograms divided by the square of the height in meters. The ratio of waist circumference to hip circumference was measured to estimate the centrality of body fat. Body composition such as fat mass and percentage fat content was measured by bioelectric impedance analysis. Impedance analysis was performed with InBody 3.0 (Biospace, Seoul, Korea).
HRV Measures at Rest, during Noise, and during Standing
After 5 minutes of rest in the supine position, HRV was measured for 5 minutes in the same position. For noise challenge, an audio frequency generator was used. The volume of sound for the 5 minutes’ exposure was set at 100 dB and the frequency at 2 KHz. During the noise exposure, HRV was measured in the supine position for 5 minutes. Finally, HRV was measured in the standing position for 5 minutes.
Equipment for Measuring HRV
The analysis of HRV was performed with the use of a device (SA-2000; Medicore, Seoul, Korea) that satisfies the standards of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.
Statistical Methods
SAS (ver. 6.12; SAS Institute, Cary, NC) was used for all statistical analyses. Each variable was checked for normality of distribution using the Shapiro-Wilk test. Of the HRV measures, SDNN, RMSSD, LF, and HF, which had significantly rightward-skewed distributions, were log-transformed to produce normalized distributions.
Values were presented as mean, standard deviation, median, and range for demographic variables and mean when analysis of covariance (ANCOVA) was used. Two-sample t tests, Wilcoxon rank sum test, and Pearson and Spearman correlation were used to determine whether the demographic and clinical variables were associated with HRV measures. To detect the differences of HRV measures among the 3 states, the repeated measures by ANCOVA and Bonferroni multiple comparison method were used to guard against an increase in type I error level.
The subjects were divided into 2 groups, separated at the median splits by percentage fat content. Repeated measures by ANCOVA models with one repeated factor (stimuli challenges) and gender, age, and heart rate as covariants were used to determine the effects of those factors.
Results
Obesity Indices of the Participants
Basic values and subjects’ obesity indices are presented in Table 1. The mean fat mass was 17.0 ± 6.0 kg, mean percentage fat was 26.3 ± 7.8%, mean waist/hip ratio was 0.89 ± 0.05, and mean BMI was 24.3 ± 3.3 kg/m2.
Characteristics of Participants (N = 41)
Participant’s Characteristics and HRV at Rest
The association between baseline HRV and participants characteristics is presented in Table 2.
Participant’s Characteristics and Heart Rate Variability at Rest (N = 41)
Age
SDNN and RMSSD were decreased with increasing age (r = −0.42, r = −0.53; P < .01). LF and HF were also negatively related with increasing age (r = −0.54, r = −0.46; P < .01). However, the LF/HF ratio did not change (r = −0.09, P > .05).
Sex
LF was significantly higher in men (P < .05). Other variables, such as SDNN, RMSSD, and HF, were higher in men, although not significantly (P > .05).
Heart Rate
SDNN, RMSSD, LF, and HF were decreased with increasing heart rate (r = −0.44, r = −0.62, r = −0.39, r = −0.53; P < .01). On the contrary, LF/HF ratio was positively related to heart rate (r = 0.31; P < .01).
Obesity Indices and HRV at Rest
The association between obesity and HRV is also presented in Table 3. There was no correlation between HRV and BMI (P > .05). By contrast, a higher level of fat mass, percentage fat, and waist/hip ratio were significantly associated with lower LF (r = −0.34, r = −0.43, P < .01; r = −0.33, P < .05) and lower RMSSD (r = −0.33, r = −0.35, r = −0.38, P < .05).
Obesity Indices and Heart Rate Variability at Rest (N = 41)
HRV Changes during Standing and Noise According to Percentage Fat (after Adjusting for Age, Gender, and Heart Rate)
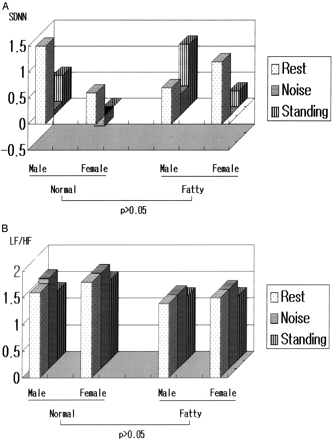
Changes of HRV response to stimuli, according to obesity, are shown in Figure 1. All results were adjusted according to age, gender, and heart rate. The median percentage fat content, 26.3%, was used as the cutoff point for the normal (51.2%) and obese (48.8%) groups, respectively.
HRV changes in standing and noise according to percent fat (after adjusting for age, gender, and heart rate).
The change in SDNN was not different (P > .05) between normal and obese group at rest and during noise and standing after adjusting for age, gender, and heart rate (normal group: male, 1.6 ± 0.2, 1.7 ± 0.1, 1.3 ± 0.2; female, 1.8 ± 0.2, 1.8 ± 0.2, 1.5 ± 0.2; obese group: male, 1.4 ± 0.0, 1.4 ± 0.0, 1.2 ± 0.0; female, 1.5 ± 0.2, 1.5 ± 0.2, 1.2 ± 0.2; P > .05).
In addition, the change amount of LF/HF ratio was not different (normal group: male; 1.5 ± 0.6, 0.1 ± 0.4, 0.6 ± 0.4, female; 0.6 ± 0.0, −0.2 ± 0.3, 0.0 ± 0.4, fatty group: male; 0.7 ± 0.0, 0.4 ± 0.0, 1.2 ± 0.0, female; 1.2 ± 0.6, 0.0 ± 0.5, 0.3 ± 0.5; P > .05).
Discussion
Significant correlations were observed between HRV and obesity indices at rest. During the resting state, higher levels of fat mass, percentage fat content, and waist/hip ratio were significantly associated with the lower LF variable, which mainly reflects sympathetic activity. The sympathoadrenal system is widely assumed to play a major role in the pathophysiology of obesity because of the regulation of energy expenditure.35 RMSSD, which reflects parasympathetic activity, was also negatively related to obesity indices in our study. This result suggests that an obese person might have an autonomic function disturbance in parasympathetic activity as well as in sympathetic activity. Among the investigators who have studied the relationship between obesity and autonomic function, there have been marked differences in opinion as to how to interpret the results.12–18 One of the main views is that obese people have a higher sympathetic tone, which is proved from elevated catecholamine levels. Another is that obese people have lower sympathetic tone, which is proved from lower LF. In our opinion, these differences of interpretation may arise from confusion concerning the meaning of HRV and sympathetic hormones. HRV is one of the efferent limbs of the autonomic nervous system, not the whole autonomic nervous system function, whereas sympathetic hormone is one of the afferent limbs of the autonomic nervous system. Because of these differences, a higher sympathetic hormone level, as indicated by catecholamine, and a lower sympathetic response activity, as indicated by LF, can be simultaneously observed in an obese person.
Our results showed that obese persons have different HRV at rest but not during stimuli. This means that higher cardiovascular disease in obese person is caused mainly by their different HRV responses at rest state, but not to stimuli. However these results also may be from the intensity or type of stimuli. Therefore, further study is needed in the future.
Many factors that affect HRV have been considered controls. HRV is a valuable tool for risk stratification in cardiovascular disease, but the physiologic effects of many other factors must also be taken into account.
Age was considered. There was a significant correlation between HRV variables and age in our study. All the cyclic components responsible for variability, which were presented by SDNN, decreased with increasing age. LF and HF, which mainly reflect sympathetic and parasympathetic activity, respectively, also decreased with increasing age. This result suggests that both parasympathetic and sympathetic activity decrease with increasing age.
Sex was also considered in our study. Men had a significantly higher LF than women. Therefore, it can be conjectured that men have greater sympathetic activity than women. This higher sympathetic activity could explain why cardiovascular disease is more prevalent in men than women. However, the mean age difference between genders (male, 43 years; female, 46 years) could also explain this result.
Heart rate was also considered. SDNN, RMSSD, LF, and HF were negatively related with increasing heart rate. On the contrary, LF/HF ratio was positively related with heart rate (r = 0.31; P < .01). This is reasonable because increased heart rate is related elevated sympathetic activity.
Although number of subjects in our investigation was small, this is an important study because it dealt with the HRV response to stimuli in obese persons.
Conclusion
Although there was no significant HRV response to stimuli in obese persons, RMSSD (which reflect heart rate variability) and LF (which mainly reflects sympathetic activity) were negatively correlated with fat mass, percentage fat content, and waist/hip ratio at rest in obese persons. These results mean obesity can change cardiac autonomic nervous response, meaning that the mechanism by which obesity increases cardiac mortality would be explained, at least partially.
- Received for publication October 15, 2004.
- Revision received October 15, 2004.