Diabetes and mental disorders are common chronic illnesses in the United States, affecting more than 16 million1 and 44.3 million Americans,2 respectively. As a result of the ever-increasing incidence of obesity, diabetes is being diagnosed more often, not only in the adult population but also in children. Additionally, more focus is being given to behavioral disorders in recent years, and the Healthy People 2010 mental health initiative is being favorably reviewed and accepted in the medical community. Consequently, such conditions as bipolar and major depression are being diagnosed and treated more often in the primary care setting. Recently, the introduction of the new class of atypical antipsychotic medications has been a major treatment advance for patients with affective disorders. These agents are being prescribed for the treatment of behavioral disorders because they provide clinically effective therapies without some of the side effects that are associated with older agents, such as the phenothiazine class of antipsychotic medications. These side effects often include extrapyramidal symptoms, of which several can be irreversible.3
Because of the increased use of the atypical antipsychotic medications, new and unanticipated side effects have appeared. Specifically, atypical antipsychotic drugs have been associated with new-onset diabetes and weight gain.1–4 These side effects have involved such agents as clozapine, olanzapine, quetiapine, and a combination of clozapine and quetiapine. The development of diabetes has been reported to occur anywhere from 10 days to 18 months after starting therapy. One theory is that diabetes might result from the weight gain caused by these agents.4–6 Other studies suggest that these agents affect glucose transport metabolism peripherally in patients, possibly increasing the potential for hyperinsulinemia and peripheral insulin resistance.7 ,8 Further hypotheses point to the activity of atypical antipsychotic drugs at the serotonin receptors of the beta cells in the pancreas, more specifically 5HT1A and 5HT2 receptors. This activity might lead to derangement of beta cell function, with resulting increases in glucose levels in patients.
A case is described of a patient who developed new-onset diabetes shortly after starting an atypical antipsychotic drug. This case differs from previous ones in that we report the temporal association of the medications and onset of diabetes, and we offer possible explanations for the onset of diabetes and management techniques from a primary care standpoint.
Case Report
A 45-year-old woman of Caribbean descent with a history of bipolar disorder, hypertension, hyperlipidemia, and obesity came to the office for follow-up of an urgent care clinic visit. Her bipolar disorder had previously been treated with several medications, including antipsychotic drugs. The patient initially began to have symptoms of polyuria, blurred vision, and general malaise. She went to a hospital-controlled screening clinic in a local area shopping mall, where her blood glucose level measurement was 375 mg/dL, and she was encouraged to seek emergency care. She went to a local urgent care clinic 2 hours later complaining of dizziness, blurred vision, and polyuria. At that time, her blood glucose level was greater than 600 mg/dL. The patient was given insulin and started on a combination of glyburide and metformin. Glyburide was started at 5 mg twice a day, and the metformin was started at 500 mg twice a day.
For the next 3 days the patient’s blood glucose level did not register below 311 mg/dL on a home blood glucose monitor. On the third day, the patient began to have symptoms of nausea, malaise, and chills. Her capillary blood glucose level was 311 mg/dL. She reported to an area teaching hospital emergency department with a chief complaint of “general ill feeling,” at which time her blood glucose level was 285 mg/dL. The emergency department physician increased the dosage of glyburide to 10 mg in the morning, and 5 mg in the evening and increased the metformin dosage to 1,000 mg in the morning and 500 mg in the evening. The next day, her blood glucose levels ranged from 234 mg/dL to 292 mg/dL.
The patient continued to experience symptoms of malaise and was seen in her primary care physician’s office. At that time, her fasting blood glucose levels were in the 300-mg/dL range. A physician’s assistant examined the patient and increased her medications to 1,000 mg of metformin twice daily, and 10 mg of glyburide twice daily. She was referred to the diabetic education service in the clinic. During a follow-up visit 3 days later, her primary care physician noticed that she was taking quetiapine and risperidone, and that she had started taking quetiapine just before the onset of her diabetes. The physician suspected that the quetiapine might be a causative factor in her illness, notified her psychiatrist of the problem, and asked the psychiatrist to consider discontinuing the medication.
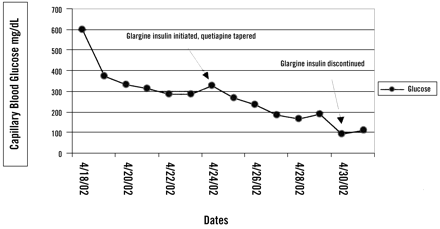
Even with the combination of antidiabetic agents, the patient’s fasting blood glucose levels continued to be elevated, and her symptoms of hyperglycemia required the addition of insulin 1 week after her initial visit to the urgent care clinic. The patient was referred to the clinical pharmacist for diabetic medication counseling and instruction in taking insulin. She started with 15 units of insulin glargine at bedtime and was given a tapering schedule for her quetiapine, because we suspected it was the offending agent (Figure 1). The daily dosage of quetiapine was reduced by one half every 2 days until the quetiapine was discontinued. As the quetiapine dosage was reduced, a subsequent decrease in daily glucose levels was observed, requiring concomitant decreases in the insulin dosage. Two weeks after the diagnosis of her diabetes, the patient’s capillary blood glucose level was 111 mg/dL in the early evening, and the insulin was discontinued.
Capillary blood glucose levels during treatment for diabetes mellitus and discontinuation of quetiapine.
Of note, the patient reported that she had maintained a consistent weight during the entire treatment regimen with quetiapine and even experienced weight loss during the initial phase of diabetes onset. Because of the patient’s bipolar disorder and the successful control of her condition with quetiapine, the psychiatrist instituted a trial of ziprasidone, 20 mg twice daily, several weeks after the patient stopped taking the quetiapine. She did not experience a return of the previously observed diabetes symptoms. The patient had previously been given other agents, including lithium, valproic acid, mirtazapine, and haloperidol, all of which had to be discontinued because of various adverse effects. The swift intervention of medication management in this patient prevented serious effects from the diabetes episode, without loss of control of the patient’s psychiatric condition.
Discussion
Antipsychotic drugs, in particular many of the new atypical antipsychotic medications, have been associated with new-onset diabetes and weight gain.1–4 We report the case of a middle-aged woman with bipolar disorder who developed new-onset diabetes after concomitant use of two antipsychotic agents, risperidone and quetiapine. The risperidone was prescribed first, approximately 5 months before the diabetes event. The patient was noted to have had a blood glucose level of 118 mg/dL after she started taking risperidone, but her blood glucose levels remained in the impaired fasting glucose range (<125 mg/dL). The natural course of the diabetes was impressive in that onset occurred so quickly after she started taking the second medication, quetiapine, which resulted in highly elevated blood glucose levels.
The natural course of the diabetes onset secondary to antipsychotic drugs is not well understood, although there are several proposed mechanisms by which it might have occurred. One possibility is the disruption of glucose transport into peripheral tissues. In animal models, researchers have been able to elucidate a mechanism by which the antipsychotic drugs markedly decreased glucose transport into target cells.7 This animal study suggests that the antipsychotic drugs might block glucose accumulation directly at the level of the glucose transporter (GLUT) protein in both peripheral and brain tissue, leading to hyperglycemia. Another possibility is a derangement of pancreas function, more specifically beta cell function.7 ,9 Pancreatic function might become depressed in the presence of the newer atypical antipsychotics.7 Compared with phenothiazine medications, the atypical antipsychotic drugs interact with greater affinities at the 5HT receptors (5HT2A and 5HT2C) in the pancreatic beta cells, which might in turn decrease pancreatic beta cell response to elevations in blood glucose.
The newer atypical antipsychotic drugs have found favor because of their improved adverse event profile with respect to extrapyramidal side effects.2 ,10 Existing predictors of type 2 diabetes mellitus should be taken into account, however, before prescribing such agents. These predictors include family history, obesity (body mass index >28), hypertension, elevated cholesterol levels, and previously recognized impaired fasting glucose (glucose levels between 110–125 mg/dL). Impaired fasting glucose, hypertension, and elevated cholesterol make up elements of metabolic syndrome, and thus predispose patients to type 2 diabetes mellitus.11 Our patient had many of the aforementioned predictors and thus was a candidate for more prudent monitoring of type 2 diabetes mellitus from the onset of antipsychotic drug therapy. The lack of noticeable weight gain during the treatment phase with quetiapine, coupled with the swiftness of the diabetes onset and progression, suggests that antipsychotic-drug–induced weight gain was not the cause of the patient’s diabetes. Our patient did not experience additional weight gain during this treatment phase with risperidone and quetiapine, as has been reported in several other case reports.
Primary care providers are often faced with the dilemma that a particular medication might cause a subsequent health problem which also requires treatment with medications. This possible outcome stresses the importance of recognizing the subtle differences that can exist among medications, even within the same drug class. As with our patient, not all medications within a class will produce a class effect with respect to benefits or adverse effects. When her treatment regimen changed from quetiapine to ziprasidone, her diabetes subsided without a loss of control of her affective disorder. Greater attention should be paid to published case reports and drug-labeling changes issued by the Food and Drug Administration or the parent company.12 In some instances, adverse effects cannot be avoided, and there is no alternative but to treat the problems caused by the offending agent. This route, however, should not be pursued, if possible. A thorough knowledge of the potential adverse effects associated with medications can offer the physician several pharmacological options to tailor treatment to an individual patient profile.
Conclusions
Although it is well-known and supported in the current literature that antipsychotic therapy can induce diabetes mellitus in patients, the primary care physician must be especially aware of its potential occurrence, because that physician must subsequently manage the patient’s glucose status following induction of treatment. Recognition of existing risk factors for diabetes, such as family history, metabolic disorder, and central obesity, is essential in the possible prevention of new-onset diabetes in patients treated with atypical antipsychotic medications.
- Received for publication December 11, 2002.
- Revision received December 11, 2002.