Abstract
Introduction: Transcutaneous bilirubin (TcB) measurement in newborns has been studied extensively in the non-Hispanic population, but its usefulness in the Hispanic population remains unclear. We evaluate the accuracy of TcB measurements in assessing jaundice in Hispanic neonates by using total serum bilirubin (TSB) measurements as the reference standard and determine the TcB level that can be used to identify neonates at risk for clinically significant jaundice (above the 95th percentile).
Methods: A total of 192 Hispanic neonates were enrolled. TcB measurements were performed within 30 minutes of obtaining the TSB measurement. The linear relationship between TcB and TSB was measured by using the Pearson correlation coefficient (r). We calculated sensitivity, specificity, and positive and negative predictive values by using a TcB level above the 75th percentile to identify neonates with a TSB level above the 95th percentile.
Results: TSB ranged from 1.7 to 13.9 mg/dL, with 62% falling below the 75th percentile. TcB correlated well with TSB in Hispanic neonates (r = 0.87). A TcB level above the 75th percentile detected all infants with a TSB level above the 95th percentile, sensitivity 100%, and specificity 66%.
Conclusions: In Hispanic newborns, the TcB level correlated well with the TSB level. TcB monitoring is a useful clinical screening tool to evaluate for the risk of clinically significant jaundice.
Neonatal jaundice, or hyperbilirubinemia, is a common problem encountered in the newborn nursery, occurring in approximately 65% of all full-term babies with the peak bilirubin levels occurring on day 5 of life.1 Complications of hyperbilirubinemia, such as acute bilirubin encephalopathy and/or kernicterus, are rare in infants whose bilirubin levels are below the 95th percentile. However, the preventability of these complications has led to recommendations to screen all neonates for hyperbilirubinemia.1–4 In July 2004, the American Academy of Pediatrics issued a clinical practice guideline, “Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of Gestation.” This guideline recommended that all newborn nurseries have a protocol for assessing jaundice. However, it did not specify the method by which jaundice must be assessed. Both TSB and TcB are cited as acceptable ways to assess neonatal jaundice.4
Levels of TcB can be determined with a device that noninvasively estimates TSB levels by measuring light transmission through the skin of neonates. There are several TcB meters available, and previous studies of the various meters have shown that a linear relationship exists between TcB and TSB [r = 0.87 to 0.96].5–10 However, at high levels of TSB (>15 mg/dL), the accuracy of TcB is less clear, but seems to be good.6,10 The literature does support using TcB as a tool for the initial assessment of neonatal jaundice as a means of decreasing the number of blood draws performed on neonates. If the TcB is high, a subsequent blood draw is then required for confirmation.5–14
The accuracy of TcB meters has been assessed in neonates from a variety of ethnic backgrounds including, African, Asian, and Indian.15–18 However, the data on Hispanic neonates are limited. Although Hispanic neonates were included in most studies, they comprised only a small percentage of the total study populations.5–7,10
Only 2 studies in the literature include a large percentage of neonates of Hispanic origin. The first study published in 2000, by Engle et al,19 found a poor correlation between TcB measurement and TSB levels in Hispanic neonates in the newborn nursery setting. However, this study focused on neonates with very high TSB levels (31% had TSB ≥15 mg/dL). A second study by the same group was published in 2005,20 this time focusing on Hispanic neonates in the outpatient setting. This study found a better correlation of TcB and TSB, but again included a large percentage of the neonates with very high TSB levels (47% had TSB ≥15 mg/dL). Thus, the usefulness of TcB measurements in general population of newborn Hispanic infants remains unclear.
This study had 2 primary objectives: 1) To evaluate the accuracy of TcB measurements for assessing jaundice in the general population of Hispanic neonates, by using TSB as the reference standard; and 2) to determine the TcB level that can be used to identify neonates who are “at risk” for clinically significant jaundice, with risk defined as a TSB level above the 95th percentile.
Methods
Setting
The study was conducted in the newborn nursery at Maricopa Medical Center, a publicly funded district hospital in Phoenix, with approximately 3500 deliveries a year. The Maricopa Medical Center newborn nursery admits all healthy infants born at the hospital who are more than 35 weeks gestation and weigh more than 2267 g. During the period of enrollment for this study (January through April 2006), approximately 800 infants were admitted to the Maricopa Medical Center nursery. More than 90% of infants admitted to the Maricopa Medical Center nursery are of Hispanic ethnicity, the majority of which are of Mexican descent.
The study was approved by the institutional review board at Maricopa Medical Center. Parents of all children received a written explanation of the study in both English and Spanish. Because this study was of a noninvasive intervention, the institutional review board required that verbal consent be obtained. The parents of all children in the study gave verbal informed consent.
Subject Eligibility
An infant was eligible for enrollment in the study if: 1) the infant was of Hispanic ethnicity, 2) the infant had not previously had a TSB level measured as part of this study, and 3) a trained nursery nurse was available to check a TcB measurement within 30 minutes of drawing a TSB level.
Data Collection
Demographics
Demographic data were collected by review of the infant's medical record. Hispanic race/ethnicity was verified by the race designation on the admission face sheet (as stated by the patient's mother at the time of registration) and/or the mother's primary language being designated as Spanish on the neonatal admission record.
TSB Measurement
All infants admitted to the MMC newborn nursery routinely undergo measurement of TSB before discharge from the nursery. TSB is measured earlier than the day of discharge if jaundice is suspected by clinical examination.
For TSB measurements, blood was obtained by venous puncture. TSB assays were performed in the clinical laboratory at Maricopa Medical Center using the Ortho VITROS 950 or the Ortho VITROS 5.1 FS Chemistry System (Ortho-Clinical Diagnostics, Rochester, NY). These analyzers measure TSB using a modified diazo reaction. The machines were calibrated daily according to the manufacturers’ recommendations. The assays used by these machines were previously studied in comparison with the gold standard bilirubin measurement of high-profile liquid chromatography and were found to correlate well.21 In addition, one study compared high-performance liquid chromatography, modified diazo reactions, and TcB measurements and found good correlation among all 3 methods.6
For TcB measurements, we used the BiliCheck device. All measurements were performed with a single BiliCheck device in accordance with the manufacturer's recommendations. The BiliCheck TcB meter has been studied extensively.6,7,9,12,15,16,19 BiliCheck measures TcB using the entire spectrum of visible light (380 to 760 nm). White light is transmitted through the skin of the neonate and by analysis of the light reflected back from the neonate's skin, an internal microprocessor calculates the amount of bilirubin present in the skin. The microprocessor uses an algorithm to account for interfering factors such as hemoglobin, melanin, and dermal thickness. As a result, theoretically, the BiliCheck should be able to provide an unbiased measurement of TcB independent of race/ethnicity.22
As noted, newborn nursery nurses obtained TcB measurements within 30 minutes of the TSB. At the onset of this study, all nurses obtaining TcB measurements received one-on-one instruction regarding the manufacturer's recommended method for obtaining TcB measurements. The device was calibrated before each measurement according to the manufacturer's recommendations. A total of 3 TcB measurements were obtained from each infant's forehead and these were averaged to obtain a single TcB measurement. The intraclass correlation coefficient for the 3 consecutive TcB readings for each infant was 0.87 (P = .000), indicating strong test-retest reliability. Therefore, the average of these 3 measurements was used in subsequent analyses. We obtained only one TSB level and one averaged TcB for each infant.
Risk Level Determination
We used the bilirubin nomogram developed by Bhutani et al3 to divide bilirubin levels into 2 groups, clinically significant jaundice, and nonclinically significant jaundice. The bilirubin nomogram plots age in hours vs. bilirubin. As the level of bilirubin normally varies with age, this nomogram helps determine whether the level of bilirubin at a particular hour of life puts an infant at risk for developing clinically significant hyperbilirubinemia. If a bilirubin value is above the 95th percentile for age curve, the nomogram predicts that the infant is in the “high-risk zone” for developing clinically significant hyperbilirubinemia. Similarly, bilirubin levels between the 75th and 95th percentile curves predict the infant is in the “high-intermediate risk zone.”
Both the TSB and the averaged TcB values obtained for each infant were plotted on this nomogram. Because the TSB and TcB values for each individual infant were obtained at essentially the same time, the same hour of life was used to plot both values. For clinical significance, we used TSB values above the 95th percentile and TcB values above the 75th percentile.6,19 All bilirubin samples were obtained during the ages of life that are included in the nomogram (0 to 144 hours old).
Data Analysis
Previous research demonstrated a high correlation (r ∼0.90) between total serum bilirubin and transcutaneous bilirubin measurements in non-Hispanic infants. To account for the possibility that the correlation may be slightly less in Hispanic neonates, we based our sample size calculation on the assumption that there would be a correlation of 0.85 between serum and transcutaneous levels in Hispanic infants. Assuming this 0.85 correlation, and assuming 95% confidence (2-tailed) and 80% power, 190 patients were needed for this study.
Data were entered into a Microsoft Excel spreadsheet and analyzed using SPSS version 14.0.
The overall relationship between TcB and TSB measures was assessed using the Pearson product moment correlation (r), regression slope (β), and Bland and Altman error plots.23 Sensitivity, specificity, and positive and negative predictive values were calculated.
Results
Subjects
A total of 198 admitted infants were enrolled. Six of these infants were eventually excluded from analysis when it was found that their race/ethnicity was not Hispanic. In total, therefore, 192 infants were included in our study.
Mean gestational age was 39 weeks and mean birth weight was 3369 g. Mean age at the time when measurements were taken was 40 hours.
Correlations
TSB values ranged from 1.7 to 13.9 mg/dL, with 62% of neonates below the 75th percentile for age, 31% with levels between the 75th and 95th percentiles for age, and 6% with levels above the 95th percentile for age (Table 1).
Sample Characteristics
TcB correlated well with TSB (r = 0.87, 95% confidence interval [CI] = 0.84 to 0.89). Fig. 1 shows the regression plot of TSB and TCB. The slope (β) of the regression plot is 0.97, illustrating the strong correlation between TcB and TSB. Overall, TcB slightly underestimated TSB, as suggested by the small offset, or Y intercept of the regression plot (y = 0.27).
Total serum bilirubin versus transcutaneous bilirubin measurements. N = 192 comparisons. The line of equality (TcB = TSB) is represented by the dotted line. The equation for the least squares best fit line is TSB = .271 + .974 (TcB), represented by the solid line.
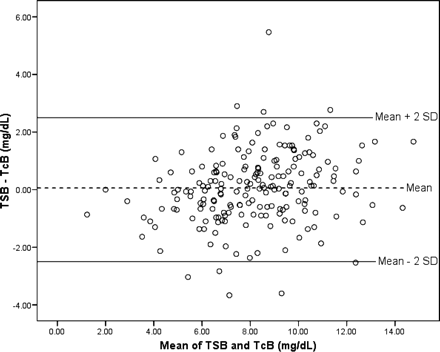
The average error in evaluating hyperbilirubinemia with TcB, compared with evaluation with TSB (calculated by TSB − TcB), was 0.06 mg/dL (95% CI = −0.12 to 0.23). A Bland-Altman error plot shown in Fig. 2, illustrates that only 9 data points (5%) fall outside 2 standard deviations of the difference, indicating that the differences are normally distributed.
Error distribution plot of total serum bilirubin and transcutaneous bilirubin. Mean difference between TSB and TcB, plotted against mean of TSB and TcB.
Sensitivity and Specificity
None of the 119 infants with TcB below the 75th percentile for age had clinically significant hyperbilirubinemia (TSB above the 95th percentile for age). The sensitivity of TcB measurements for detecting this level of hyperbilirubinemia was thus 100%. A total of 73 infants had TcB above the 75th percentile for age. Of these, only 12 had clinically significant jaundice with TSB level above the 95th percentile for age. Thus, the specificity was 66% for detecting clinically significant hyperbilirubinemia (Table 2).
Predictive Indices Using >95th Percentile TSB and ≥75th Percentile TcB*
Discussion
The BiliCheck TcB meter has been studied extensively,6,7,9,12,15,16,19 but its usefulness in the Hispanic population became questionable with the publication of a study done in 2000 at Parkland Hospital by Engle et al.19 This study found that there was a poor correlation between TcB measurements and TSB levels in Hispanic neonates. However, because the Engle study included a relatively large percentage of infants with very high TSB levels (≥15), the usefulness of TcB measurements in the general population of Hispanic, most of whom would have a normal or mildly elevated bilirubin level, remained unclear.
Our study investigated the correlation of TcB levels with TSB levels in the Hispanic population with the intent of using TcB levels as a screening tool for clinically significant (above the 95th percentile) neonatal hyperbilirubinemia. According to our results, in the Hispanic population, as in other ethnic groups, TcB levels correlate well with the gold standard measurement of TSB. Furthermore, our study demonstrated that using a TcB level of above the 75th percentile for age was 100% sensitive in identifying infants who may be at risk for clinically significant hyperbilirubinemia.
As early discharge from the hospital is becoming more frequent, there has been increasing concern that infants may develop serious sequelae of unrecognized hyperbilirubinemia. This concern has led to a number of recommendations for universal screening for neonatal hyperbilirubinemia, and ultimately resulted in the publication of the 2004 American Academy of Pediatrics guideline for management of neonatal hyperbilirubinemia.2,3 The guideline recommended a predischarge risk-assessment for the development of severe hyperbilirubinemia in every newborn. At the time the guideline was published, transcutaneous bilirubinometry had already been developed, but its use was not universal. With the call for universal screening the value of an effective, safe, and noninvasive tool for the evaluation of neonatal hyperbilirubinemia grew.
The results of our study support the use of the TcB measurement as a first-line tool for hyperbilirubinemia screening. If an infant has a TcB level above the 75th percentile for age, it is safe to assume that the infant has a low risk of developing clinically significant hyperbilirubinemia, and no further testing need be performed. In our nursery, where every newborn is required to have a TSB level checked before discharge, this would reduce the number of TSB measurements, and hence a potentially painful blood draw, by 60%. In addition, the use of TcB as a first-line screening tool also might result in a significant health-care cost reduction. At the time of the study, the average cost (charged to the patient) of a TcB and TSB was $4 and $15, respectively (a 73% cost difference).
Limitations
Our study has several limitations that should be considered when interpreting the results.
First, because of nursing staff considerations, the infants in our study were not enrolled consecutively or randomly. Instead, infants were only enrolled in the study when appropriately trained nursing staff was available. Because the infants were not sampled consecutively, the potential for selection bias is possible. The magnitude and direction of this bias, if it occurred, cannot be determined.
Next, because our study focused on normal newborns, the results of this study may not be applicable to Hispanic neonates who are preterm, have low birth weight, or have other risk factors for hyperbilirubinemia such as infection.
In summary, the results of this study support the use of the transcutaneous bilirubin meter as a screening tool for clinically significant hyperbilirubinemia in Hispanic infants. The TcB meter had excellent sensitivity, making it unlikely that an infant with a significant level of hyperbilirubinemia would escape detection with TcB measurement. Although the specificity of the TcB level was only 66%, the test was able to identify a sizeable group of infants that did not need invasive testing for the evaluation of hyperbilirubinemia. Based on our results, we recommend that all normal, term, Hispanic infants be screened for hyperbilirubinemia initially with the measurement of TcB level. If the results indicate a TcB level above the 75th percentile for age, a TSB level should be obtained.
Acknowledgments
We acknowledge the support of the nursing staff of the newborn nursery at Maricopa Medical Center and their assistance with data collection.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
- Received for publication September 30, 2006.
- Revision received January 8, 2007.
- Accepted for publication January 11, 2007.