Abstract
Objective: The objective of this study was to adapt the National Diabetes Prevention Program (N-DPP) into a pragmatic tool for primary care settings by using daily text messaging to deliver all N-DPP content, supplemented by Fitbit technology to provide behavioral strategies typically delivered by personnel in traditional programs. Test the mobile health (mHealth), technology-based N-DPP adaptation (DPPFit) in primary care patients with prediabetes using a remote intervention based on the traditional 16 core sessions of the DPP.
Methods: A pilot study with pre/post survey analysis of aggregate data were used to determine changes in weight, physical activity, sedentary behavior, and associated diabetes risk outcomes among study participants (n = 33). In this study, participants were issued Fitbit devices and provided the remote intervention over 16 weeks via automated text messaging technology, which followed the content of the DPP core education sessions.
Results: Data analysis from baseline to 6-month follow-up demonstrate mean weight loss of 3.3 kg (95% CI: -6.2 to -0.5; P = .026), reduction in body mass index by 1.25 points (95% CI: -2.1 to -0.4; P = .005), a significant average increase of 2 days in self-reported physical activity per week (95% CI: 0.4 to 3.6; P = .015) and an average 10% decrease in sedentary time (P = .007).
Conclusions: The remote DPPFit intervention demonstrates a promising and practical approach to the management of prediabetes in a primary care setting. The results support the use of the DPPFit program and application to achieve meaningful outcomes in a population with prediabetes. A randomized controlled trial with a larger sample is warranted.
- Diabetes Prevention Program (DPP)
- Lifestyle
- Metabolic Syndrome
- mHealth
- Primary Health Care
- Technology
- Telemedicine
- Translational Research
The threat of diabetes mellitus to community health, coupled with the economic impact on health systems, demands immediate attention. Type 2 diabetes mellitus (T2D) is the focus of the present study and prevention programs worldwide. In 2019, the International Diabetes Federation (IDF) estimated that 463 million people had diabetes, with 90% having T2D.1 The projected prevalence of diabetes worldwide is expected to grow to 578 million by the year 2030.2 The prevalence of those at-risk of developing T2D is expected to grow to 8% (454 million) by 2030.2 This forecast is alarming.
Lifestyle modifications can prevent or delay the onset of diabetes,3⇓–5 more effectively than metformin in the Diabetes Prevention Program (DPP) trials.5 The DPP's individualized and resource-intensive strategies showed that a modest 7% decrease in body weight was significantly related to reduced diabetes incidence, where each kilogram of weight loss is equal to a 16% diabetes risk reduction.6 Moderate physical activity greater than or equal to 150 minutes a week was the second strongest negative predictor of diabetes incidence.6
Following the risk reduction results of the DPP, the US Congress authorized the Centers for Disease Control and Prevention (CDC) to establish a National Diabetes Prevention Program (N-DPP) to provide communities across the United States as a form of the evidence-based program.7 With this congressional mandate, the CDC established and monitors the Diabetes Prevention Recognition Program (DPRP; https://nccd.cdc.gov/DDT_DPRP/Registry.aspx), a formal registry of N-DPP programs nationwide.8 Although the CDC's initiative evolves and includes more than 1 acceptable curriculum, the 16 core sessions from the DPP trials serve as the foundation of all N-DPP curricula and the current adaptation.7 The efficacy demonstrated in the DPP, and through the N-DPP, remains a challenge to implement in primary care, where both personnel and time limit implementation of diabetes prevention initiatives.9–10 Where resources are an issue, N-DPP adaptations for primary care and other real-world settings are not reaching the DPP-established benchmarks for weight loss.9
In 1999, the cost for the first year of the DPP trials was estimated at $1,399 per participant, with some cost reductions in group delivery of the N-DPP program.11 The most significant barrier, and expense, to adapting DPP delivery to the community practice setting is the reliance on personnel to deliver the intervention material and serve as the model and reinforcer of performance accomplishments in goal setting.12 The common theme is the need for someone other than the patient to be responsible for the prevention program delivery. Unfortunately, these personnel intensive strategy impedes our ability to meet the growing needs of community care and patient populations.13 The present study seeks to determine the necessity of personnel to deliver the N-DPP by instead adapting the full lifestyle intervention to mobile health (mHealth) delivery. This adaptation uses consumer wearable devices (Fitbit®) in combination with the mobile Fitbit® application (app) to recognize performance accomplishments,12 support goal setting, and afford self-monitoring of food and physical activity by the individual.
mHealth technologies offer an opportunity to assist both family medicine patients and clinicians to address prediabetes by enacting diabetes prevention steps in the family medicine clinic. Rapid advances have occurred in relatively low-cost wearable devices that assist consumers in monitoring their physical activity and becoming more active.14⇓–16 A growing body of research has successfully incorporated fitness devices into technology-oriented lifestyle interventions to increase physical activity, reduce obesity, and manage chronic health conditions.17⇓–19 This automated tracking and recording of physical activity reduces the burden of traditional self-monitoring and provides an ongoing record of performance accomplishment, the most influential source of individual self-efficacy.12 The present adaptation leverages fitness tracker technology and the accompanying apps to reduce the burden of manually tracking food and physical activity. Further, by relying on the technology to provide user feedback and SMS/MMS to deliver the intervention content, DPPFit may be the first adaptation of the N-DPP that does not use personnel to deliver the intervention or behavioral strategies (e.g., logging performance accomplishments or providing feedback about performance).
This study's primary purpose was to adapt the DPP, and subsequent N-DPP, into a pragmatic tool for clinical settings by using automated text messaging to deliver all written content, supplemented by Fitbit® technology to provide behavioral strategies typically delivered by personnel in traditional programs. The secondary purpose was to test the feasibility and acceptability of the mHealth, technology-based N-DPP adaptation (DPPFit) among patients at risk of developing T2D. The remote 16-week intervention delivered the same content, behavioral guidance, and education of the 16 DPP core sessions.
Research Design and Methods
Overview of Study Design
The present pilot of this intervention used a pretest/post-test design, assessing changes in participants from baseline to the 6-month follow-up.
Study Population and Setting
All 33 participants in the study were patients at a general internal medicine primary care clinic in Augusta, Georgia. Recruitment occurred from December 2019 through January 2020, using medical records, primary care manager (PCM) referral, or in response to a recruitment flyer posted in the clinic. All patients had been told they had prediabetes and were interested in how to reduce their risk of developing T2D.
Prediabetes status was defined as HbA1c values 5.7%–6.4% and/or fasting plasma glucose (FPG) 100–125 mg/dL.20 Exclusion criteria included a history of diabetes, bariatric surgery, prior or current medication use to treat glucose intolerance (e.g., biguanides or sulfonylureas), or prescription weight loss pharmaceuticals. Women with a history of gestational diabetes mellitus who met all other criteria were not excluded. All patients had to be cleared by their PCM for participation and were excluded for any comorbidities that increased risk during physical activity (e.g., chronic heart failure or history of heart disease). Participants had to have a smartphone device to use throughout the study.
Baseline visits were conducted by the first author and the research team in the clinic, where potential participants were consented and issued a Fitbit® activity tracker. At baseline, the Fitbit® was set up by an investigator, and each participant downloaded the Fitbit® mobile app to his or her personal cell phone. Demographics, socioeconomic status, anthropometric measures, and survey instrumentation were collected at baseline for all consenting participants. To account for anyone diagnosed with T2D during the study, diabetes history and diagnoses were queried in baseline and post-test surveys.
DPPFit Intervention
The foundation of the N-DPP is a 12-month program focused on 2 primary goals: losing weight (5%–7%) and being active (150 physical activity minutes/week). The first 6 months of the intervention focus on core concepts of the prevention program delivered in 16 sessions. The goal of the present research was to follow the 16 core sessions of the DPP by providing the program content in a more pragmatic way through SMS/MMS text messages delivered daily to participants. For example, week 1 of the intervention includes daily SMS/MMS messages corresponding to the DPP Participant Workbook Session 1.
All consenting participants agreed to receive daily text messages over the next 16 weeks, which would include hyperlinks to additional information or media files in addition to text. An outline of the 16-week intervention and session topics, which followed the core sessions of the DPP, were provided to participants at baseline. Participants were enrolled on a rolling basis. The DPPFit intervention was delivered remotely over the ensuing 16 weeks and utilized technology-based methods to substitute for the behavioral strategies delivered by lifestyle coaches (e.g., reinforcement of performance accomplishment).
Fitbit Technology
The role of the wearable technology was to supplement the SMS/MMS daily messages so that the behavioral strategies from the DPP could more fully be realized in this remote intervention format (See Table 1). One example of how the Fitbit® app refines certain DPP activities is logging and tracking food. In the traditional DPP, participants are instructed to look up fat gram counts and record their food consumption. In DPPFit, participants were instructed to enter food consumption in the Fitbit® app, where fat and calorie intake were automatically calculated. Table 1 details how wearable technology and the Fitbit® mHealth app were used to reinforce and supplement evidence-based behavioral strategies.
Behavioral Strategy Adaptations from National-Diabetes Prevention Program to DPPFit
Participants received a 16-week technology–based intervention that used wrist-worn Fitbit® Blaze™ physical activity monitoring devices (San Francisco, CA) paired with the accompanying Fitbit® app, a mobile phone app that connects to the wearable device. As the app collected information from the wearable throughout the day, participants could monitor minutes of physical activity and received instant feedback on their activity through the Fitbit® app. Daily, Fitbit® users could record food items and beverages consumed in an electronic food diary as part of the Fitbit® app. Users were able to track minutes of physical activity per day enabling them to receive feedback on their activity. Fitbit ‘how-to’ hyperlinks related to setting goals, tracking activity, and logging food were provided via SMS/MMS messaging. This content supplemented educational content from the DPP core sessions and was delivered along the timeline of the DPP scope and sequence. The research team instructed participants to wear the trackers throughout the study and to call the study team for any technical assistance. This study did not obtain outcome data from the Fitbit® devices or app. The wearable technology acted as a feedback mechanism for participants to know if they were meeting personal goals, which would inform participant perceptions of mastery and self-efficacy. In this way, the fitness tracker and Fitbit® app were substitutes for personnel to deliver and support behavioral components of the DPP. The participant interaction with and use of the wearable technology were treated as confidentially as any interactions would be with a health coach or clinician.
To our knowledge, this is the first use of the DPP intervention that did not use personnel, remote or in person, to deliver the 16-week content. As such, the method used to deliver the DPP program content was central to this technology-based adaptation. The messaging platform allowed for storage of group and template content and scheduling future messages.
Project Broadcast©
Project Broadcast© is one of several mass media apps marketed to allow users to send mass SMS/MMS texts (i.e., broadcasts) to contacts anonymously between contacts and by generating a new number where the messages will originate. The platform also allows users to create messaging templates that can be used to preschedule messages, in this context, a total of 16 weeks of messages scheduled in advance. For purposes of this study, broadcasts sent Monday to Friday were scheduled for 9:00 am local time, while Saturday and Sunday messages were at 10:00 am local time. The messaging app provides delivery confirmations of outgoing messages but not read receipts. To confirm the sending of the prescheduled text messaging templates (SMS/MMS), a study team member was included in each of the two start dates. This also allowed the study team to view how the message content appeared to recipients. The Project Broadcast© app was independent of the wearable technology and mHealth app used by participants and was only used by the study team to deliver the educational content. A template designating each day of the 16-week study was developed and saved in the Project Broadcast© app (See Figure 1). A total of 113 templates were created. In addition to the 112 days of the study (16 weeks by 7 days a week), a DPPFit_0 template notified participants the day before the start of the intervention to save the number to their phone contacts (See Figure 2). See Table 2 for examples of the message content and how they supported the overall behavioral strategies. A complete list of the messaging templates is available as Online Appendix.
Screenshot of messaging templates in project. Broadcast App.
Screenshot of DPPFit days 0 and 1 messages.
Use of Technology and mHealth App to Deliver Behavioral Strategies*
Intervention Cost
The cost to transmit each daily SMS/MMS message is $0.02. The messaging platform's total cost for 113 days of messages (1 is welcome text; the next 112 days are the 16-week intervention) is $2.26 per participant. All Fitbit® devices use the same Fitbit® app interface, which is freely available. Therefore, the Fitbit® wearable cost is the primary cost for each DPPFit participant. The newest Fitbit® trackers range from $69.95 for the Inspire 2™ up to $179.95 for the Charge 5™. Some clinic patients may already own a fitness tracker.
Instrumentation
During the baseline visit, participants completed a survey, including questions about socio-economic demographics, medical history, and self-reported physical activity.21 Total minutes and of physical activity and, independently, sedentary time were collected from the survey, as well as days a week of physical activity by type (i.e., vigorous, moderate, walking), defined as at least 30 minutes of activity on that day. Any day of the week where at least 30 minutes of activity were reported The Finnish Diabetes Risk Score (FINDRISC) was used to classify diabetes risk after the baseline visit.22–23
FINDRISC was selected for its extensive validation studies among patients with prediabetes and T2D.22⇓–24 Scores range from 0 to 20 and were calculated for each consenting participant using information collected from the electronic medical record and baseline biometric/survey measures.22–23 Consistent with prior research, a cutoff of 9 was used to determine those at risk, where 9 to 12 was considered moderate risk with 2.2% 10-year risk of developing T2D, and ≥13 was considered high risk with a 14.1% 10-year risk of developing T2D.22 This metric allowed us to classify participants as high or moderate risk based on the most widely utilized, low-cost, methods available.23
Statistical Analysis
All statistical analysis was performed using SAS 9.4 software (SAS Institute). Statistical significance was assessed using an α level of 0.05. Descriptive statistics for all variables were determined including frequencies and percentages for categorical variables, means and standard deviations and 95% confidence intervals (CI) for continuous variables, and medians and interquartile ranges for ordinal variables. A repeated measures mixed model analysis was used for examining differences from pre- to post-intervention in weight, physical activity, and sedentary behavior.
Results
Baseline measures of diabetes risk, demographics, and socio-economic status are displayed in Table 3. Overall, the majority of participants were female (82%), African American (49%), had completed undergraduate or graduate degrees (76%), were employed (76%), and partnered or married (73%).
Baseline Measures of Diabetes Risk, Demographics, and Socioeconomic Status
The measures of diabetes risk in Table 3 include anthropometric and clinical characteristics for the 33 study participants. The mean age was 44.4 years (standard deviation [SD] ± 8.5), with an average FINDRISC mean of 13.6 (SD ± 2.6), where 64% of the participants were considered high-risk and 36% were moderate.23 Mean hemoglobin A1c level was 5.9 (SD ± 0.28).
Controlling for demographics and FINDRISC, the main outcomes are summarized in Table 4, including changes in weight, body mass index (BMI), blood pressure, self-reported physical activity, and sedentary behavior. There was a significant decrease from baseline to 6-month follow-up in total weight (-3.3 kg, P = .026) and in overall BMI (-1.25 kg/m2, P = .005).
Pre- and Post-Intervention Effect: Biometrics and Self-Reported Physical Activity Outcome from the International Physical Activity Questionnaire
Table 4 also shows results for the physical activity outcomes from baseline to follow-up. There were several statistically significant increases in physical activity. There was a 2 day increase in moderate days of physical activity a week between baseline and follow-up (95% CI: 0.4 to 3.6; P = .015) and a 1.5 day increase in vigorous days a week of physical activity (95% CI: 0.1 to 2.9; P = .035) between baseline and follow-up. There were increases in physical activity minutes per week (62 minutes, P = .039), as well as days a week of both moderate (2 days, P = .015) and vigorous (1.5 days, P = .04) activity. There was a significant decrease in sedentary time, from 509.5 minutes per day at baseline, to 388 minutes per day at follow-up (P = .007).
Discussion
The novelty in the present pilot of DPPFit is that it delivers the content of the N-DPP 16-week intervention without the need for dedicated personnel (e.g., educators, lifestyle coaches). The combination of the daily text messages of DPP content and use of the Fitbit® to deliver behavioral strategies may provide meaningful changes in health outcomes such as weight loss, increased activity, and decreased sedentary time. Several meta-analyses have attempted to quantify this gap between clinical trial efficacy and real-world effectiveness.9,10,25⇓–27 Dunkley et al concluded that at the same follow-up time point, real-world adaptations have had a third of the impact on weight outcomes as the DPP, and about half of the Finnish Diabetes Prevention Study's weight outcome.26 Thus, DPPFit could exceed these outcomes in a practical, scalable way to reach many people.
In a 2012 meta-analysis by Ali et al, which included only high-risk participants from the United States, the mean percentage of weight change from the 26 studies (n = 2916) was -4% (95% CI: -5.2 to -2.8).11 In another meta-analysis of 22 studies (n = 5500) which was not limited to the United States,10 the -2.6% weight change was like the -3% change in weight in the present study. Even a 1 kg loss in weight may significantly reduce the risk of diabetes incidence by 16%.6 While only 19% of the DPPFit cohort met the goal of ≥5% of total weight loss, 69% of those that did not meet the weight loss goal still achieved the physical activity goal.
Independent of weight loss, increasing moderate physical activity to the recommended ≥150 minutes a week reduces the risk of future diabetes incidence.6 Reporting of physical activity is not as consistent in DPP translations as the outcome of weight, and often varies in its expression of activity (i.e., days per week; minutes per day per week). For the 69% of the DPPFit sample that did not meet the weight goal, but did meet the physical activity goal, they could see a 44% lower incidence of diabetes.6
Independent of physical activity, several studies have documented the link between sedentary lifestyles and metabolic disorders, insulin resistance, and subsequent diabetes incidence.28⇓–30 Efforts to increase physical activity do not equate to decreases in sedentary time. It is important to demonstrate this independence from physical activity goals, in establishing sedentary time reductions as significant to overall diabetes risk reduction.28⇓–30 In the measurement of sedentary behavior across all 3 treatment arms in the DPP clinical trials, researchers found that for each hour spent watching television, the risk of developing diabetes increased by 3.4% (hazard ratio = 1.034, 95% CI: 1.004–1.065). Sedentary time reduction was not an original goal of the DPP trials, nor was any aspect of the intervention specifically designed to address sedentary behaviors.30 The significant decrease in sedentary time in the DPPFit study may be a result of the short follow-up time but could result from the use of wearable devices that alert users to move.
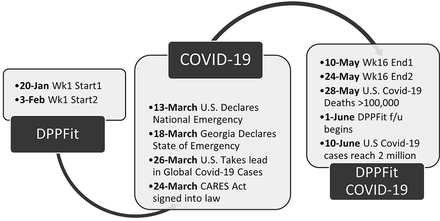
DPPFit was a response to meet the needs of physicians attempting to address diabetes prevention for their patients. It is clear now that DPPFit may also meet the needs of a population at risk for diabetes who are unable to attend traditional prevention programs. One strength of this study was a fortuitous intersection of methods and timing. The 16-week remote pilot study of DPPFit started just before the COVID-19 pandemic affected the United States (See Figure 3). By mid-March 2020, the United States and the state of Georgia had declared a public health emergency. The COVID-19 pandemic has created opportunities for remote work, telemedicine, and technology-based approaches to contactless care.31⇓–33 As a result, Americans are increasingly interacting virtually, reducing face-to-face interactions. The DPPFit intervention is remote and designed to require minimal contact to meet the reality of time constraints on family medicine clinics. The timing of this intervention through the emergence of the COVID-19 pandemic demonstrated the ability of virtual intervention delivery to persist through a public health emergency.
Timeline of the DPPFit intervention and the Covid-19 pandemic in the US.
As a nonrandomized pilot study, selection bias is a study limitation. Participants were recruited either by referral from their PCM, or if identified by their medical record data and inclusion criteria, they were cleared for participation by the PCM. Access to medical records was approved for recruitment purposes only, so there was no analysis of those enrolled versus. others who were recruited but did not enroll. While the COVID-19 pandemic did not disrupt the intervention delivery, the study was limited in how and when follow-up data were collected. For example, the study did not describe whether participants had previously used Fitbits or were at ease with the mobile technology. Another limitation concerned the self-report of the baseline and follow-up survey instruments. The present study cannot measure the impact of sustained behaviors over time. Follow-up research should extend beyond 6 months and assess sustained behavior change at regular intervals (i.e., 12, 18, and 24 months). Further research is needed to address these limitations through use of a randomized controlled design, more thorough screening, surveying participants on prior use and comfort with technology, and understanding participant use after the intervention ends.
Some limitations were cost-reducing methods, and therefore are also strengths of the present study. Following consent into the study, the FINDRISC provided a more comprehensive diabetes risk index23 without the use of costly glucose tolerance-based testing. This use of a validated risk test is both a strength for lowering the overall cost of the intervention and a limitation in terms of the absence of clinical measures of glucose tolerance and risk.
Leveraging technology as a substitution for personnel effort and resources may also be a cost-saving method to delivering the DPP in family medicine patients. The integration of Fitbit® technology is particularly appealing as there are dozens of Fitbit devices that are compatible with its app, allowing users with varying levels of purchasing power to take advantage of the Fitbit mHealth interface. It is clearly a strength that this intervention costs as little as $102.21 ($2.26 text messages plus $99.95 Fitbit device) with a fitness tracker and requires less time effort from family medicine clinic staff, lowering the cost of personnel resources. Clinic patients already using Fitbits will only need to sign up to receive the automated text messages, at a cost of $2.26 per patient. For comparison, the CDC estimates the average cost of participation in the N-DPP around $417 per person.34
In conclusion, the outcomes of the initial pilot study demonstrate a scalable strategy for the N-DPP as DPPFit and provide practical tools for clinicians and communities. As an adaptation, DPPFit is not intended to compete with the N-DPP classes, but rather parallel these initiatives and offer options to patients unable to connect with existing programs. The remote and technology-based format of DPPFit may be suitable as an alternative option where barriers to access or participate in the N-DPP render it inaccessible.35 The results from this study suggest that remote dissemination of intervention materials, supplemented by activity and food tracking technology, may be both a feasible and acceptable way to deliver diabetes prevention in primary care settings.
Acknowledgments
Steven S. Coughlin, PhD served as the faculty advisor of this research study and continues to contribute mentorship and guidance to advance this important work. We would also like to thank the co-investigators on the original study who were not involved in this manuscript, but were nonetheless significant contributors: Jack Ellis, DO; Judith Anglin, PhD; and Jennifer Waller, PhD.
Appendix: DPPFit Daily Message Content
Notes
This article was externally peer reviewed.
Funding: This study was not part of any extramural funding opportunity and has no funding to declare.
Conflict of interests: CJWL is Assistant Editor of the JABFM.
To see this article online, please go to: http://jabfm.org/content/35/3/548.full.
- Received for publication October 20, 2021.
- Revision received February 8, 2022.
- Accepted for publication February 14, 2022.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- 27.
- 28.
- 29.
- 30.
- 31.
- 32.
- 33.
- 34.
- 35.