Abstract
Background: Glucagon-like peptide-1 agonists (GLP-1a) and sodium-glucose cotransporter-2 inhibitors (SGLT-2i) are recommended in carefully selected patients with type 2 diabetes. This study will assess prescription of these medications and investigate predictors of prescription.
Methods: This retrospective cross-sectional study included 31,354 patients. Data including sociodemographic descriptors, clinical histories, medications, and health insurance providers were extracted from a health system's administrative databases. Variables to be associated with prescription of a GLP-1a or SGLT-2i were assessed using a multivariable logistic regression model.
Results: Mean age was 62.58 years and 40.8% identified as African American. Only 3.4% were prescribed a GLP-1a and 2.1% received a SGLT-2i. Logistic regression demonstrated lower odds of receiving either medication in the highest age-group (70 to 79 years) (GLP-1a: odds ratio [OR] 0.44, P < .01, SGLT-2i: OR 0.39, P < .01) and in African Americans (GLP-1a: OR 0.64, P < .01, SGLT-2i: OR 0.28, P < .01). Atherosclerotic cardiovascular disease was not associated with GLP-1a prescription (P = .54) and conferred lower odds of being prescribed SGLT-2i (OR 0.68, P < .01). History of chronic kidney disease conferred lower odds of receiving GLP-1a and was not associated with the odds of receiving SGLT-2i.
Conclusions: Prescription of GLP-1a and SGLT-2i medications was low as compared with existing literature. Advanced age and African American race were negatively associated with prescription of these medications. Contrary to guideline recommendations; atherosclerotic cardiovascular disease and chronic kidney disease were not positively associated with prescription.
- African Americans
- Cross-Sectional Studies
- Glucagon-Like Peptide 1
- Logistic Models
- Obesity
- Pharmacoepidemiology
- Prescriptions
- Retrospective Studies
- Social Determinants of Health
- Socioeconomic Status
- Sodium-Glucose Transporter 2 Inhibitors
- Type 2 Diabetes Mellitus
The American Diabetes Association recommends the use of certain glucagon-like peptide-1 agonists (GLP-1a) and/or sodium-glucose cotransporter-2 inhibitors (SGLT-2i) in carefully selected patients as second-line pharmacologic therapies to achieve glycemic control in patients with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD).1 The American Diabetes Association also recommends certain SGLT-2i as the preferred second-line pharmacologic therapy in heart failure with reduced ejection fraction or chronic kidney disease (CKD).2 These guidelines were influenced by the results of cardiovascular safety trials mandated by the Food and Drug Administration (FDA) in 2008 before approval of new antidiabetic medications.3 Several large randomized-controlled trials have documented the safety of these antihyperglycemics and showed positive outcomes in major adverse cardiovascular events4⇓⇓–7 leading to FDA label indications for cardiovascular risk reduction. Beyond these FDA label indications, there are numerous studies that have demonstrated the safety and cardiovascular benefit of GLP-1a medications.8⇓⇓⇓⇓–13
Despite these advances, a “lag-time” in the translation of novel therapies into wide-spread clinical application has been described.14 It has been suggested that it may take more than a decade for prescribers to widely adopt drugs into their practices despite existing evidence of benefit.14 Historically, the delay to adopt the initiation of β-blockers or aspirin after myocardial infarction are some of the starkest reminders of this lag.15
In addition, literature has described disparities in prescribing practices based on patient demographics and the prescriber's specialty and gender.16,17 For example, African American participants in a 2017 study were found to be significantly less likely to be prescribed multiple myeloma treatments that yield superior outcomes.18 Furthermore, Schore et al found that African American patients pay more in provider fees, while receiving less therapy intensification when indicated.19 Prescribing practices of novel antihyperglycemics may face similar obstacles.
A recent large scale retrospective analysis described US national prescribing practices for antihyperglycemic agents. This study indicated that although the use of GLP-1a or SGLT-2i as second-line agents gradually increased leading up to liraglutide and empagliflozin receiving FDA label indications for cardiovascular risk reduction in 2016, these drug classes were used significantly less than their alternatives such as sulfonylureas, dipeptidyl peptidase 4 inhibitors and insulin.20 There is limited literature examining what factors may predict utilization of GLP-1a and SGLT-2i medication classes. This study aims to determine existing prescription practices for GLP-1a and SGLT-2i medication classes, as well as identify any demographic or medical history factors that suggest increased likelihood of prescription of these medication classes.
Methods
Data Source
A sample population was identified from the administrative databases of a large tertiary-care health system in metropolitan Detroit (Wayne, Macomb and Oakland counties). Data were extracted from the linked electronic health records for the identified patients.
Study Design
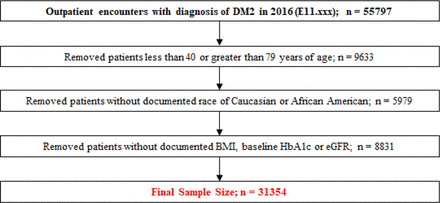
With institutional review board approval, a retrospective cross-sectional study was developed. The initial population included 1) adult patients between ages 40 to 79 years; 2) who had an outpatient encounter with an International Classification of Diseases, Tenth Revision, diagnosis code for type 2 diabetes mellitus (E11.xxx) in 2016. Patients were excluded when there was no documentation confirming 1) white or African American race; or 2) measurements of body mass index (BMI) in 2016 and another 12 to 18 months later, measurements of hemoglobin A1c (HbA1c) in 2016 and another 12 to 18 months later and estimated glomerular filtration rate (eGFR) within 12 months of January 1, 2016. A total of 31,354 eligible patients were included in the analysis. Patients included in the final population represented patients seen in either a primary care or subspecialist setting. Arrival at the final study population is summarized in Figure 1.
Attrition diagram for study population sample selection. Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; DM2, diabetes mellitus type 2.
Measurements of Main Exposure and Outcome Variables
Age was categorized as 40 to 49, 50 to 59, 60 to 69, and 70 to 79 years. BMI was categorized based on World Health Organization criteria (underweight < 18.5 kg/m2, normal 18.5 to 24.9 kg/m2, overweight 25 to 29.9 kg/m2, obese 30 to 39.9 kg/m2 and morbidly obese ≥ 40 kg/m2). HbA1c was categorized into 3 strata (HbA1c ≤ 7.0% [≤ 53 mmol/mol], 7.1%-8.9% [54 to 74 mmol/mol] and ≥ 9.0% [≥ 75 mmol/mol]). Additional covariates included race, gender, eGFR, prescription medications, median income by zip code, type of health insurance carrier; as well as relevant past medical history including hypertension, ASCVD and tobacco use status. History of ASCVD was defined as the presence of any International Classification of Diseases, Ninth Revision or Tenth Revision, diagnosis code for coronary artery disease, ischemic stroke, peripheral artery disease, myocardial infarction, or Current Procedural Terminology procedure codes for coronary revascularization procedures (ie, percutaneous coronary intervention and coronary artery bypass surgery). Reported eGFR was calculated using the CKD-EPI equation. Median household income was obtained by mapping patients' addresses to census block group level median household income. Insurance coverage was reported in groups defined as commercial, Medicaid or Medicare.
Statistical Analyses
The study population was described using mean and standard deviations for continuous variables and frequencies for categorical variables. A multivariable logistic regression model was generated to examine the associations between covariates and the outcomes of receiving a prescription for GLP-1a, SGLT-2i, or both. Bivariate analysis was conducted to examine the data, however selection of variables for the logistic regression model was not based on bivariate outcomes. Independent variables were selected for inclusion in the model based on authors' judgment of variables likely to correlate with the outcome categories (prescription of GLP-1a or SLGT-2i). A main effects model was utilized and the absence of a prescription for any of the outcome categories (GLP-1a or SLGT-2i) was designated as the reference category. Variables were introduced using a forward entry method. Considering that continuous variables were entered into the model as grouped categorical variables, an assessment of linearity was not performed. All analyses were performed using SPSS Statistics 26 (IBM, Armonk, NY).
Results
Sample Characteristics
Clinical characteristics and medications of the 31,354-patient sample are summarized in Table 1.
Baseline Characteristics
Bivariate analyses were used to explore the characteristics associated with use of GLP-1a or SGLT-2i. Although gender was not associated with use, white race, commercial insurance, younger age, and residence in higher income areas were associated with greater use. Clinical characteristics associated with greater use included higher BMI, higher HbA1c, lower CKD stage and the use of statins, metformin and/or insulin. Persons with a history of ASCVD were less likely to receive GLP-1a or SGLT-2i. Lastly, current cigarette use was associated with reduced use of GLP-1a or SGLT-2i. A detailed report of bivariate analysis results is available in the Appendix.
GLP-1a
The logistic regression model demonstrates that the odds of prescription of GLP-1a were lower for older patients (ie, aged 70 to 79 as compared with ages 40 to 49 [odds ratio [OR] 0.44, 95% CI 0.33-0.59; P < .01]). Furthermore, odds ratio decreased progressively with each increment in age category. Odds were lower for males (OR 0.72, 95% CI 0.63-0.82; P < .01) and African Americans (OR 0.64, 95% CI 0.55-0.74; P < .01). No significant odds were attributed to history of ASCVD (OR 0.95, 95% CI 0.82-1.11; p 0.53). Odds of GLP-1a prescription were higher for patients on insulin (OR 2.30, 95% CI 1.54-3.44; P < .01), higher HbA1c levels, and for patients with median household incomes > $66,000 as compared with the reference category (Table 2).
Logistic Regression GLP-1a
SGLT-2i
Odds of prescription of SGLT-2i were similarly lower for older patients (aged 70 to 79 as compared with ages 40 to 49; OR 0.39, 95% CI 0.27-0.57; P < .01). Odds were also found to be lower in African American patients (OR 0.28, 95% CI 0.23-0.35; P < .01) and in patients with history of ASCVD (OR 0.68, 95% CI 0.55-0.84; P < .01). Odds were higher for patients on insulin (OR 1.25, 95% CI 1.06-1.45; P < .01) and metformin (OR 1.21, 95% CI 1.01-1.47; P < .04), as well as for patients with higher HbA1c (7.1%-8.9% [54 to 74 mmol/mol], OR 2.27, 95% CI 1.87-2.76; P < .01; ≥ 9.0% [≥ 75 mmol/mol], OR 2.32, 95% CI 1.86-2.91; P < .01). Other covariates including gender, BMI, eGFR, smoking status, income or health insurance carrier were not found to be significant (Table 3).
Logistic Regression SGLT-2i
Discussion
Compared with the national diabetes prevalence of 8.5% reported by the Centers for Disease Control and Prevention in 2016, the prevalence of diabetes in the counties from which the sample population was drawn (Wayne [10.3%], Oakland [7.7%] and Macomb [9.5%]) was similar or greater.21 Despite this, the study population showed lower prescription of GLP-1a (3.4%) and SGLT-2i (2.1%) as compared with the national-level prescription rates in 2016 (7% and 7%, respectively).20 The logistic regression analysis offers suggestions as to the nature of this disproportion.
In both GLP-1a and SGLT-2i analyses, older age produced significantly lower odds of receiving prescriptions for either of these medication categories. Within a cohort of the general US population aged 40 to 79, those in the oldest quartile (70 to 79 years) accounted for 14.4%.22 The same age-group in the study sample accounted for 27.0% of participants, suggesting an overall older population. By comparison, when examining the national population with type 2 diabetes, Montvida et al reported 22% of patients being more than 70 years old at the time of initiation of their second antidiabetes drug.20 With increasing age, clinical factors such as advanced CKD (eGFR < 30 mL/min), higher goal HbA1c and increased sensitivity to adverse effects may guide prescribers away from the use of GLP-1a or SGLT-2i.23 However, other indications for the use of these medication classes, such as ASCVD or heart failure with reduced ejection fraction, become more common with increasing age.
African American race was the only other variable to produce lower odds of prescription in both GLP-1a and SGLT-2i analyses. The study population included 40.8% African American participants whereas national US Census data describes 12.7% of respondents identifying as African American in 2016.24 This discrepancy may suggest rationale for the differences in medication prescription in the study population as compared with Montvida et al's analysis of national prescription patterns, which also reported a 12% population of African American participants.20 A meta-analysis of recent cardiovascular safety trials for GLP-1a, SGLT-2i and dipeptidyl peptidase 4 inhibitor medications found no significant difference in the incidence of major adverse cardiovascular events as compared with placebo for the African American participants (n = 4601 [4.4% of total pooled trial participants]) of these trials.25 Another meta-analysis of African American participants in 7 liraglutide trials demonstrated benefits in improved glycemic control and weight loss as compared with placebo.26 Review of this existing literature on the use of GLP-1a or SGLT-2i medications in African American populations provide insufficient data on preventing adverse cardiovascular outcomes in African American population.
Residence in a zip code with greater median household income correlate with higher odds of GLP-1a prescription (similar analysis for SGLT-2i did not reach significance). Those residing in neighborhoods where the median household income was ≥ $66,000 had greater odds of being prescribed a GLP-1a when compared with the reference group (< $27,000).
Neither the presence of CKD nor ASCVD increased the likelihood of prescription of these medication classes in the study population. Interestingly, participants with ASCVD were found to have lower odds of being prescribed an SGLT-2i and participants with CKD stages 2, 3a or 3b had lower odds of being prescribed GLP-1a. In years since the study period, treatment guidelines have been substantially updated to recommend the use of these agents in individuals with diabetes and either CKD or ASCVD.27 Investigating the uptake of these recommendations may be an interesting area for future study.
In summary, this retrospective cross-sectional study finds that the prescription of GLP-1a and SGLT-2i medications was low when mainstream clinical guidelines began advocating their use. This study also indicates that age, race, sex and income impact the likelihood of being prescribed these novel medications. Further study may reveal how GLP-1a and SGLT-2i prescription has changed since contemporary guidelines have promulgated further.
Limitations
Related to use of a retrospective cross-sectional chart review design, this study was limited to data reliably found in medical records. By extension, data regarding diet, exercise, medication side effect and adherence to prescription could not be included. Income data were collected from a population level data source (census block-group data) whereas all other data were individual level (electronic health record/health system administrative database). Data regarding the proportion of primary care vs subspecialist prescribers was not collected/examined.
Although, a strength of the study population was the inclusion of a sample with 40% of participants identified as African American, this also presents a limitation in its generalizability nationwide. Furthermore, it is acknowledged that participants without a documented race or a race other than white or African American categories were not included in the study.
Acknowledgments
We thank Chandpreet Sohi, DO, Kemal Bahcheli, MD, Hege Heggum, MD, and Della Rees, PhD, for their assistance with the study.
Appendix
Bivariate Analysis for Use of GLP-1a or SGLT-2i
Notes
This article was externally peer reviewed.
Funding: None.
Conflicts of interest: None.
To see this article online, please go to: http://jabfm.org/content/35/2/256.full.
- Received for publication August 26, 2021.
- Revision received November 5, 2021.
- Accepted for publication November 9, 2021.