Abstract
Objective: To provide primary care providers an up-to-date approach to the diagnosis and management of atopic dermatitis (AD).
Quality of Evidence: PubMed, Cochrane, and MEDLINE databases were searched for relevant meta-analyses, randomized controlled trials, systematic reviews, and observational studies about the diagnosis and management of AD.
Main Message: AD is a common chronic skin disease characterized by immune dysregulation and skin barrier dysfunction. It has a substantial impact on quality of life across all ages. Despite this, AD is often still undertreated due to inaccurate diagnoses, even by many experienced dermatologists. Many primary care providers may not be aware of its commonly associated medical and psychiatric comorbidities or be familiar with new treatment options.
Conclusion: In this clinical review, we describe the pathophysiology, epidemiology, diagnosis, and treatment of AD, specifically highlighting commonly used therapies and novel medications.
Background
Atopic dermatitis (AD) is a common and chronic inflammatory skin disorder.1,2 In North America, the prevalence ranges between 9.8% and 10.3% in children ages 6 to 7 and 6.5% and 9.4% in ages 13 to 14.3 Close to three-quarters of cases obtain complete remission by age 16.4 However, adult prevalence remains around 10%.5
AD arises from an interplay between environmental exposures, epidermal barrier dysfunctions, and immune dysregulation. There is no single genetic cause of AD; however, mutations in filaggrin seem to play an important role.6,7 Filaggrin deficiency weakens skin barrier integrity and increases epidermal permeability, thereby predisposing individuals with AD to irritant and allergic reactions.8⇓–10 In children, FLG mutations positively correlate with development of atopies, such as rhinitis and asthma,7 which form the “atopic triad.”
Risk factors for AD include a family history of asthma (odds ratio [OR], 1.38; 95% confidence interval [CI], 1.10-1.59), allergic rhinitis (OR, 1.33; 95% CI, 1.14-1.54), or AD (OR, 2.4; 95% CI, 1.89-3.05).11 Passive tobacco smoke seems to be associated with AD.12 There is a higher prevalence of AD in urban than in rural environments.13 An increased maternal intake of probiotics14,15 may have a modest benefit and prevent AD.
Clinical features
AD is characterized by ill-defined, erythematous, and scaly plaques. The unaffected skin may have pruritus and xerosis.8,16 Acute lesions are characterized by erythematous papulovesicular papules and plaques,8,16 subacute lesions by poorly defined scaly erythematous patches, and chronic lesions as lichenified plaques.8,16 AD can have different patterns in different age groups. Infant AD is characterized by head and neck and extensor limb surface involvement; the trunk may also be affected, but the diaper area is usually spared.8,16 Children present with a shift toward chronic lesions on the flexural folds around the wrists, ankles, hands, feet, and antecubital and popliteal fossae; there is a lower frequency of facial involvement in children than in infants.8,16 Adult AD usually affects the flexural areas and has a greater variety of subtypes, including those that exclusively affect the hands, nipples, genitalia,17 and/or eyelids.8,16
The most commonly used diagnostic criteria for AD include 4 components: (1) pruritus, (2) dermatitis in infant or adult distributions, (3) chronic or relapsing dermatitis, and (4) personal or family history of atopy.18 Twenty-three minor criteria, grouped into facial features, triggers, complications, and other (Table 1)18 support an AD diagnosis. The presence of 3 major and 3 minor criteria are required for a definitive diagnosis of AD.18,19
Diagnostic Criteria for Atopic Dermatitis18
The severity of AD is commonly categorized20,21 into mild-to-moderate AD involving limited areas of the body affected, milder intensity of pruritus, and sleep loss; and severe AD involving widespread areas of dry skin, high frequency of pruritus, and a significant impact on quality of life.
Differential diagnosis
The common differential for AD (Table 2) includes seborrheic dermatitis, irritant contact dermatitis, allergic contact dermatitis, plaque psoriasis, and scabies infestation.20,23
Common Conditions in the Differential Diagnosis of Atopic Dermatitis
Seborrheic dermatitis usually presents as ill-defined erythema with greasy scale8 in areas with a high density of sebaceous glands, such as the nose, nasolabial folds, eyebrows, glabella, retro-auricular folds, and scalp.20 In infants, seborrheic dermatitis can cause cradle-cap: thick adherent greasy scale covering the scalp.22,24
Irritant contact dermatitis and allergic contact dermatitis can present similarly to AD, with acute to chronic dermatitic lesions, but are usually confined to specific areas of exposure.8 Irritant contact dermatitis is a nonallergic irritation from direct exposure to solvents and often coexists with AD.7,20 Allergic contact dermatitis is a type IV hypersensitivity that occurs 12 to 72 hours after allergen exposure, and although it usually starts confined to the area of exposure, it is more likely to spread to surrounding areas than irritant contact dermatitis.8,20,23 Refractory cases of AD are commonly complicated by allergic contact dermatitis. Patch testing can be helpful to confirm the diagnosis of allergic contact dermatitis.
Plaque psoriasis usually presents as bright beefy-red and well-circumscribed plaques with silvery “micaceous” scale in a symmetric distribution. It most often affects the scalp, nails, genitals, and extensor surfaces.23 Inverse psoriasis may also affect the flexural sites but will lack scale due to excessive moisture.23
Scabies has a primary presentation of burrows of serpiginous tracks, which can cause a hypersensitivity and secondary dermatitis.25 It should be suspected in all patients with acute onset pruritus, and empiric treatment may be considered. In infants and children, the palms, soles, dorsal feet, genitalia, and diaper area are commonly affected; in adolescents and adults, the interdigital space, wrist, axillae, waist, umbilicus, and nipples are often involved.20,23
Rarely, malignancy such as cutaneous T-cell lymphomas can mimic dermatitis. It often affects the bathing trunk distribution and can be misdiagnosed as psoriasis or dermatitis, both clinically and on histopathology.8,20
Infectious Complications
Due to impaired barrier function and decreased skin antimicrobial peptides, patients with AD are predisposed to bacterial and viral skin infections. The 2 most common secondary infections in AD management are impetigo and eczema herpeticum.8
Secondary infection of dermatitis is most often associated with Staphylococcus aureus or Streptococcus pyogenes. It may present with yellow crusting, pustules, and/or bulla.8 S. aureus infections in AD are more likely methicillin resistant (MRSA) and produce superantigens,26 which can worsen dermatitis. Mild cases can be treated with topical antibiotics, such as 2% fusidic acid cream/ointment 2 or 3 times daily for 7 to 14 days, 2% mupirocin ointment 3 times daily for 10 days, or 1% ozenoxacin cream twice daily for 5 days. All 3 topical agents have activity against MRSA. Severe impetigo requires oral antibiotics, such as cephalexin. Cases of suspected MRSA should be treated with oral doxycycline or sulfamethoxazole-trimethoprim based on local resistance patterns.
Eczema herpeticum is a serious infection caused by latent herpes simplex virus. It presents as vesicles, punched-out erosions, hemorrhagic crusts, pain, lymphadenopathy, and/or fever.27 It can resemble and coexist with impetigo, making diagnosis challenging. Serious cases can involve keratoconjunctivitis and meningitis, leading to death.27 In suspected cases of eczema herpeticum, bacterial and viral microbiologic studies should be collected and patients should be treated empirically with systemic antiviral medication. Patients should be referred urgently to dermatology and/or the emergency department.
Comorbidities
Patients with AD may have a higher prevalence of several comorbid medical and psychiatric conditions. The more widely known and prevalent conditions include allergic rhinitis, asthma, and food allergies,28 particularly in the pediatric population. Obesity and cardiovascular conditions29 (such as myocardial infarction, unstable angina, early cardiovascular deaths, and others) have also been associated with more severe AD, likely coinciding with other lifestyle factors, such as poor sleep,30 tobacco use,31,12 and decreased physical activity.32 Further studies are required to define appropriate and validated screening modalities in AD populations.
AD is associated with several mental health conditions. A US pediatric cross-sectional study (n = 92642) reported a higher prevalence of depression (odds ratio [OR], 1.81; 95% confidence interval [CI], 1.46–2.39), anxiety (OR, 1.77; 95%, CI 1.36–2.29), conduct disorder (OR, 1.87; 95% CI, 1.46–2.39), and autism (OR, 3.04; 95% CI, 2.13–4.34) in AD patients.33 Meta-analyses have shown a higher prevalence of child and adult depression,34 as well as suicidal ideation35,36 (OR, 1.44; 95% CI, 1.25–1.65) and attempted suicide (OR, 1.36; 95% CI, 1.09–1.7).35,36 Reports of an association between AD and attention-deficit/hyperactivity disorder36 in the pediatric population have also been noted. There are also reports of independent sleep disturbances in children and their caregivers30,37 as well as adults.30
Treatment
Moisturizers
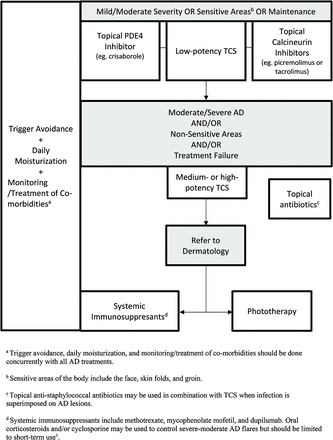
Regular use of moisturizers can both treat mild AD and prevent disease exacerbations8,38,39 and are the first-line and cornerstone of AD treatment (Figure 1). They are composed of various amounts of emollient and humectant and adjunct ingredients, such as ceramides, urea, lactate, and salicylic acid. These adjunct ingredients are physiologic lipids of the stratum corneum and act to hydrate and/or repair it, reducing pruritus, xerosis, flares, and infection.40 Ceramides comprise 50% of stratum corneum lipids41 and are of particular interest in recent AD literature. A small study (n = 80) demonstrated that prophylactic use of ceramide-dominant emollients in infants at high risk for AD for 6 months starting in the neonatal period trended toward a reduced incidence of AD at 12 months.42 However, similar effects have also been noted in the use of standard emollients.43,44 Topical coconut oil has also been used in mild-to-moderate pediatric AD.45 Although many meta-analyses regarding the use of live oral probiotics have not shown consistent improvement in AD severity,46,47 some new moisturizers may include topical prebiotic ingredients. Regardless, no particular moisturizer or application regimen has shown superiority over others in direct comparisons for AD outcomes.48,49 Moisturizers should be applied immediately after bathing or showering.42 Some studies have shown that 6% bleach baths can be used for their antimicrobial properties,50,51 but their benefit in AD has been controversial, with recent meta-analysis showing no difference with water baths.52
Treatment of AD. Abbreviations: TCS, Topical corticosteroids; AD, atopic dermatitis; PDE-4, Phosphodiesterase-4 inhibitor.
Trigger Avoidance
Some literature suggests that AD flares are seasonal and tend to correspond with periods of low humidity and low temperature.53 Patients with AD have a higher risk of irritant and allergic contact dermatitis, so common allergens such as fragranced products should be avoided.54,55 Clinicians should counsel patients on the importance of applying daily moisturizers to the entire body at least once per day and avoidance of common allergens and irritants.43
Topical Corticosteroids and Antibiotics
Topical corticosteroids (TCS) are the first-line medication56 for controlling mild-to-moderate AD (Table 3). Proper use involves considering both the potency of TCS and lesion location. Mild potency corticosteroids should be used on the face and skin folds and moderate potency corticosteroids on the body. They are typically applied twice daily for 2 to 4 weeks.39 Acute flares and thick/lichenified lesions on the body can be treated with high potency TCS (I, II) for up to 2 weeks but should then be tapered to a lower potency until the lesion resolves.39
Prescription Therapies for Atopic Dermatitis
Adverse effects of TCS include cutaneous atrophy, especially on the face and skin folds.57 For these areas, low potency formulations (VI, VII) should be used56; however, if necessary, a 5- to 7-day course of high potency steroid tapered to a lower dose is acceptable.16 As a guideline, roughly 0.5 g (1 adult fingertip) of topical steroid should be spread over 2 palms worth of skin16 and used 1 to 2 times daily,58 with liberal concurrent emollient application.8,16 If no effects are seen after 2 weeks, clinicians should consider alternative potencies or medications or consider another diagnosis.16 Due to widespread concern regarding steroid atrophy among patients and health providers, many patients underapply TCS, which can lead to decreased efficacy and inadequate symptom control.55 Patients should be counseled on adequate application for the amount and duration of medication use. Prophylactic use of low-to-moderate potency TCS can be used to control continually refractory lesions.38 Further side effects of TCS, including telangiectasia, striae, acneiform or rosacea-like eruptions, allergic contact dermatitis, and impairment of wound healing, are uncommon with appropriate use and can be effectively mananged.59
Topical antibiotics, such as fusidic acid and mupirocin, are recommended as an adjunct medication with topical anti-inflammatories when signs of infection, such as crusting, oozing, and pus, coexist with AD.60 Ozenoxacin cream (1%) is a novel topical antibiotic with activity against MRSA that was recently approved for treating impetigo in patients ages 2 months and over and is applied twice daily for 5 days.61 Corticosteroid and antibiotic combination creams, such as 0.1% fusidic acid/betamethasone valerate or 1% fusidic acid/hydrocortisone exist, but the prophylactic use of topical antibiotics on AD lesions without signs of infection is not recommended.38 Swabs for culture and sensitivity should also be considered due to the rising incidence of resistant organisms.38 At present, antistaphylococcal topical antibiotics show noninferiority to 1 another for the treatment response of infected AD lesions.61
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCIs) are nonsteroidal agents that inhibit calcineurin-dependent T-cell activation.16 TCIs may be used as an alternative treatment or for prolonged treatment when steroid atrophy is a concern.38 They may also be used as adjunctive or maintenance therapy alongside high-dose TCS.16 Two TCIs are used in practice: 1% pimecrolimus and 0.03% or 0.1% tacrolimus, with both showing efficacy in short-term (3 to 12 week) and long-term (up to 12 months) use in adult and pediatric populations. Pimecrolimus (1%) is less effective than TCS but has value as a long-term maintenance therapy, and 0.1% tacrolimus is noninferior to moderately potent TCS.62 TCIs also may be used for the prophylactic treatment of recurrent flares, applied 2 to 3 times per week to prevent relapse.16 The most common side effect of TCIs16,62 is a burning or stinging sensation over the applied area; however, this often decreases with continual use.16 They should be discontinued if allergic/contact dermatitis or rosacea-like lesions develop.16 The US FDA has a black box warning concerning the long-term safety of TCIs due to an association of systemic immunosuppression with skin malignancies and lymphoma63; however, follow-up studies have failed to verify this.64,65 Currently, the Canadian Dermatology Association does not support this black box warning66 and has recently been removed from the product monograph of pimecrolimus.67
Topical PDE-4 Inhibitors
Crisaborole (2%) ointment is a topical PDE-4 inhibitor that was approved for AD treatment by Health Canada in 2018 for patients older than 2 years.67 It has shown benefit in controlling the signs and symptoms of AD in acute and chronic lesions for both adult and pediatric populations and is well-tolerated in areas susceptible to steroid atrophy, such as the face, skin folds, and groin.68 Current safety profiles of up to 52 weeks69 show that crisaborole has a reduced risk of systemic side effects compared with TCS and TCIs.70 The most common side effect noted in clinical trials has been temporary application site burning (4.4% crisaborole vs 1.2% control; P = .001) but typically resolves after the first day of application.68,70
Phototherapy
Phototherapy is a treatment option in AD for those in which topical treatments do not achieve adequate control.71 It typically involves a 2 to 5 times weekly administration of narrowband (309 to 312 nm) UVB72 to affected areas and shows benefit in the signs, symptoms, and quality of life in AD patients.73⇓⇓⇓⇓–78 Side effects of phototherapy range from erythema, advanced skin-aging, burning, itching, and nausea.16,78 Although it is effective, phototherapy access remains an issue for many patients and the frequency of treatments can be burdensome.
Dupilumab and JAK Inhibitors
Dupilumab is a subcutaneously administered human monoclonal antibody for the interleukin-4 receptor-α subunit and is approved for the treatment of moderate-severe AD in North America and Europe.79 A meta-analysis of 8 AD trials with dupilumab showed a relative risk of skin infection of 0.54 (95% CI, 0.42-0.70) and OD of eczema herpeticum of 0.34 (95% CI, 0.14-0.84) in dupilumab versus placebo, with no significant associations with herpes simplex virus or overall skin infections.80 Patients receiving dupilumab may also be at an increased risk of conjunctivitis.81 No baseline or follow-up laboratory monitoring is required. Ongoing studies are assessing longer-term effects.82
A new class of emerging AD therapy are JAK inhibitors, which act on a common inflammatory pathway of many dermatoses, including AD, alopecia areata, psoriasis, and vitiligo.83 Currently, phase II and III studies are ongoing for topical and oral formulations.
Conclusions
The management of AD should involve the liberal use of emollients and an avoidance of common irritants. In mild-to-moderate AD, TCS are the first-line medical therapy, followed by calcineurin inhibitors, which have been shown to be noninferior to TCS in controlling AD, without the risk of steroid atrophy. Topical PDE-4 inhibitors, such as crisaborole, are a new nonsteroidal anti-inflammatory approved for ages 2 years and older. For cases of AD that fail to be controlled with topical therapy, phototherapy should be considered, if available. Newer biologics, such as dupilumab, have been approved for moderate-to-severe AD and seem to have favorable efficacy and side-effect profiles according to randomized trials.
Notes
↵# Co-first authors.
This article was externally peer reviewed.
Conflicts of Interest: PF has received honoraria and/or consulting fees and/or advisory board fees for AbbVie, Aralez, Altius, Cipher, Eli Lilly, Galderma, Janssen, Leo, Novartis, and Pfizer & Sanofi. YBY has no conflicts of interest to declare. CL has acted as a principal investigator, speaker and/or consultant and/or advisory board fees for AbbVie, Amgen AnatpysBio, Avillon, Arcutis, Bristol-Myers Squibb, Celgene Cipher Genentech, GlenMark, Incyte, Janssen, Leo Pharma, Kyowa, Pfizer, Merck Novartis, and Sanofi. BO has no conflicts of interest to declare. KOL has received honoraria and/or consulting fees and/or advisory board fees from Bausch, Eli Lilly & Pfizer.
Funding: None.
Biographical: PF is an Assistant Professor in the Division of Dermatology, University of Toronto. YBY is a senior medical student at the University of British Columbia. CL is an Associate Professor in the Division of Dermatology, University of Toronto. BO is an Assistant Professor in the Department of Family & Community Medicine, University of Toronto, North York General Hospital. KOL is a Lecturer in the Department of Family & Community Medicine, University of Toronto, St Michael's Hospital.
To see this article online, please go to: http://jabfm.org/content/33/4/626.full.
- Received for publication December 12, 2019.
- Revision received March 2, 2020.
- Accepted for publication March 4, 2020.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵