Abstract
Introduction: Beginning around 2011, innumerable policies have aimed to improve pain treatment while minimizing harms from excessive use of opioids. It is not known whether changing insurance coverage for specific conditions is an effective strategy. We describe and assess the effect of an innovative Oregon Medicaid back/neck pain coverage policy on opioid prescribing patterns.
Methods: This retrospective cohort study uses electronic health record data from a network of community health centers (CHCs) in Oregon to analyze prescription opioid dose changes among patients on long-term opioid treatment (LOT) affected by the policy.
Results: Of the 1,789 patients on LOT at baseline, 41.6% had an average daily dose of <20 morphine milligram equivalents (MME), 32.3% had ≥20 to <50 MME, 14.5% had ≥50 to <90 MME, and 11.6% ≥90 MME. Around half of each group discontinued opioids within the 18-month policy period. Those who discontinued did so gradually (average of 11 months) regardless of starting dosage. Predictors of discontinuation included: diagnosis of opioid use disorder, older, non-Hispanic white, and less medical complexity.
Conclusions: Regardless of starting opioid dose, nearly half of patients affected by the 2016 Oregon Medicaid back/neck pain treatment policy no longer received opioid prescriptions by the end of the 18-month study period; another 30% decreased their dose. Gradual dose reduction was typical. These outcomes suggest that the policy impacted opioid prescribing. Understanding patient experiences resulting from such policies could help clinicians and policy makers navigate the complex balance between potential harms and benefits of LOT.
- Chronic Pain
- Community Health Centers
- Health Policy
- Medicaid
- Opioids
- Oregon
- Physician's Practice Patterns
- Prescriptions
- Primary Health Care
- Retrospective Studies
Introduction
The U.S. problem with opioid-related morbidity and mortality has been well documented.1⇓–3 It was initially driven by opioid prescriptions from medical settings, with nearly half generated in primary care clinics.4,5 Although opioid prescribing has steadily declined since 2011, prescription rates remain historically high.6 Many policies have been implemented at federal, state, and local levels aimed at promoting safer and more efficacious prescribing of opioids. 7⇓–9
A unique opioid policy was implemented in Oregon in 2016. The state's Medicaid program is provided through the Oregon Health Plan (OHP). Using evidence synthesized by the state Health Evidence Review Commission,10,11 the OHP instituted 2 coverage guidelines that aimed to improve access to safer, nonpharmacologic evidence-based treatments while concurrently limiting payments for long-term opioid therapy (LOT) and other interventional approaches for back and neck pain.12,13
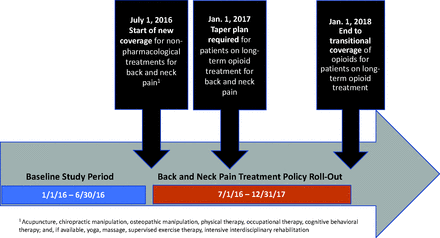
These changes were intended to be implemented step-wise over an 18-month period starting in July 2016 when the OHP began reimbursing nonpharmacological treatments, such as acupuncture, massage, and physical therapy, for back and neck pain (see Figure 1). In January 2017, clinicians were expected to establish a taper plan for patients on LOT; by January 2018 coverage of opioids for back and neck pain was restricted to a 90-day limit with the intent of ending long-term opioid treatment for back and neck pain at that time.
Timeline of Oregon policy implementation.
Unfortunately, little is known about how such an insurance payment policy might affect patient opioid use over time and particularly, what was the experience of patients on LOT. Prior experience suggests it is hard to taper patients who have been on opioids long-term, especially those on higher doses.14,15 While there is general agreement that opioids have been overprescribed in the U.S., recent literature has raised questions about potential harms of inappropriate forced reductions or tapering too rapidly.16⇓⇓⇓–20
Community health centers (CHCs) are clinics that serve predominantly low-income patients by reducing barriers to treatment such as cost. A large proportion of patients seen at CHCs are Medicaid beneficiaries who were impacted by the Oregon policy changes described above.21 Using electronic health record (EHR) data from a large network of CHCs in Oregon, we retrospectively evaluated a cohort of patients with Medicaid insurance on LOT with back and neck pain at the time the OHP guidelines went into effect (July 2016) to measure the extent to which patients were able to taper or discontinue use of opioids by the January 1, 2018 date of payment restriction. In addition, we assessed how rapidly those dose reductions took place, and differences in discontinuation rates by important sociodemographic and clinical characteristics. This study is part of a larger mixed-methods research project evaluating the impact of the Oregon Medicaid treatment reimbursement policy using a natural experiment design.22
Methods
We conducted a retrospective cohort study using EHR data from OCHIN, a nonprofit community health information network providing a linked, hosted EHR to >1,000 CHC sites across 22 states. These data are part of the Accelerating Data Value Across a National Community Health Center (ADVANCE) Clinical Research Network (CRN), a member of PCORnet. At the time of the study, OCHIN represented approximately 70% of the CHCs in Oregon, and these CHCs provided care to an estimated 20% of all statewide Medicaid enrollees. To approximate the policy's target population from EHR data, we identified a cohort of Oregon patients aged 18 to 64 with at least 1 Medicaid-insured visit and recorded diagnosis codes for back or neck pain documented in the baseline 12 months before July 1, 2016. We then “followed” this group in the EHR data for an 18-month study period, ending December 31, 2017. Inclusion criteria included having at least 1 office visit in the study period.
Patients on LOT were identified by being prescribed at least 60 pills or tablets of opioid pain medication or any fentanyl patch in each of the 2 calendar quarters before baseline (January 1, 2016–March 31, 2016 and April 1, 2016–June 30, 2016). We excluded patients with documentation of cancer or palliative care at any point in the study period, as these enrollees were excluded from the opioid reduction policy.
The eligible patient population at baseline was described demographically (sex, age, race/ethnicity) and clinically (annual visit rate, documented substance use disorders, and mood disorders). We used the Charlson Comorbidity Index23 as an aggregate measure of medical complexity at baseline, and counted the number of pain conditions in addition to back and/or neck pain in accordance with meaningful pain condition categories established by the National Pain Strategy Epidemiologic Working Group.24 All conditions were assessed from diagnosis codes recorded on patients' EHR problem lists at baseline; back and neck pain diagnoses used for study inclusion were additionally ascertained from diagnoses recorded in encounters. Problem lists are actively maintained by clinicians in this CHC network, with noted and inactive dates allowing us to ascertain conditions that were “active” for each patient at different timepoints. Condition definitions are presented in Appendix Table 1.
Baseline Characteristics of Oregon Medicaid Patients with Back or Neck Pain on Long-Term Opioid Therapy
We pulled all opioid prescribing data recorded in the EHR from July 1, 2016 through December 31, 2017 to assess tapering and discontinuation corresponding to the expected interval for tapering affected patients off LOT. Morphine milligram equivalents (MME) were calculated using standard methods and conversion factors.25 To estimate average daily MME at baseline we summed total MME prescribed for the period January 1, 2016–June 30, 2016 (the 2 quarters used to assess LOT status) and divided by 181 days. To estimate average daily MME throughout the follow-up period we summed total MME per quarter, for each of the 6 follow-up quarters, and divided by 90 days.
We categorized patients on LOT into 4 groups for analysis based on average daily MME at baseline: less than 20 MME, 20 or more and less than 50 MME, 50 or more and less than 90 MME, and at least 90 MME. At the study's end point, we described the percentage of each group who discontinued (ie, had 0 MME prescribed in last quarter or earlier), had a partial reduction in MME (ie, last quarter MME less than baseline MME), or had no reduction in MME (ie, last quarter MME equal to or greater than baseline MME). To describe patterns of reduction, we calculated median average daily dosages with interquartile ranges for each study quarter and, for those who completely tapered, the mean number of quarters to achieve 0 MME.
Finally, we estimated crude and adjusted odds ratios (aOR) of discontinuing opioid use by all demographic and clinical variables described above.
Although CHC utilization was not a primary outcome, number of visits is likely related to opioid prescribing and reduction outcomes. Thus, we also examined annual visit rates during the 18-month observation period stratified by ending dose and, for patients who fully discontinued opioid use, described visits that occurred after their last opioid prescription (Appendix Table 2). A sensitivity analysis was conducted by excluding patients who appeared to have discontinued opioid use but did not return for a follow-up visit in the study period following their last opioid prescription date (Appendix Tables 3-T4).
Opioid Reduction Outcomes Among Oregon Medicaid Patients with Back or Neck Pain on Long-Term Opioid Therapy, by Baseline Dose
Odds of Reducing Opioid Usage to Zero Among Oregon Medicaid Patients with Back or Neck Pain on Long-Term Opioid Therapy
Statistical significance was set at P < .05. Data management and analysis were conducted using SAS (v9.4; SAS Institute Inc., Cary, North Carolina). This project was approved by the Kaiser Permanente Washington Institutional Review Board.
Results
Among the total cohort of LOT patients at baseline (n = 1789), 745 (41.6%) had an average daily dose less than 20 MME, 578 (32.3%) were between 20 and 50 MME, 259 (14.5%) were between 50 and 90 MME, and 207 (11.6%) were on baseline doses of at least 90 MME (Table 1). The sample was predominantly female (60.5%) and non-Hispanic white (87.0%), with a mean age of 49.2. The cohort had a high level of medical complexity (32.5% had baseline Charlson Comorbidity Index scores ≥3; 61.4% had a diagnosed mood disorder). Patients were also high utilizers of these CHC clinics, having a median of 9 ambulatory visits in the year before the policy introduction.
Across the entire cohort, 47.9% demonstrated discontinuation of opioid use by the end of the 18-month policy period; another 30.0% reduced their daily MME dose and 20.3% experienced no reduction in dose (Table 2). Similar patterns were seen when stratified by baseline dose: 52.6% of the lowest dosage group (less than 20 MME) reduced to 0 MME, as did 47.4% of those starting between 20 and 50 MME, 48.3% of the between 50 and 90 MME group, and 47.3% of the highest dosage (at least 90 MME) group. Among the highest-dose group at baseline, only 44 patients (21.3%) remained on doses of at least 90 MME at the end of follow-up, and only 13 of these patients (6.3%) had no reduction in their average daily opioid dose. Other groups experienced similar reduction to safer doses, even among those who did not fully taper.
Patients who discontinued opioid use tapered to zero across an average of 3.6 calendar quarters (or about 11 months; Table 2); the highest dose group at baseline (at least 90 MME) tapered more slowly (mean = 4.1 quarters, or about 12 months) than groups with lower baseline doses. Gradual tapering was evident across all groups, regardless of starting or ending dosage (Figure 2).
MME reduction among Oregon Medicaid patients with back or neck pain on long-term opioid therapy by starting dose, over 18 months postpolicy implementation
Patients on the highest opioid doses at the end of follow-up had higher CHC utilization during the study period than their lower-dose counterparts, but all groups continued to have high visit rates throughout the observation period (at least 4.7 visits per year on average, Appendix Table 2). Among patients who discontinued opioid use, 64% of them returned for 1 or more visits after their last opioid prescription. When limiting to patients who returned for ≥1 visit after their last opioid prescription (sensitivity population), a smaller proportion was observed to discontinue opioid use (38.6%), 36.6% had a partial reduction, and 24.9% had no reduction in MME (Appendix Table 4). Baseline demographics and opioid reduction outcomes by starting dose followed similar patterns to the full sample (Appendix Tables 3-T4).
In adjusted analyses, the strongest predictor of LOT patients achieving a complete taper was a diagnosis of opioid use disorder: Opioid use disorder patients had 70% higher odds of discontinuation than their counterparts without this diagnosis (aOR = 1.70, 95% CI, 1.05–2.71; Table 3). Non-Hispanic White patients were more likely to be completely tapered relative to patients of other racial/ethnic groups (aOR = 1.61, 95% CI, 1.21–2.15), and older patients had lower odds of discontinuation than their younger counterparts. Patients with higher levels of medical complexity were significantly less likely to discontinue opioid use relative to those with a Charlson score of 0 (Charlson score ≥3 aOR = 0.68, 95% CI, 0.50–0.93).
Discussion
The rising prevalence of opioid overdose deaths in the U.S. was declared an epidemic in 2011;8 especially thereafter, policies were enacted at national, state, and local levels to rein in what was perceived as excessive opioid prescribing.9,26,27 The Oregon policy described here was unique in its attempt to align state Medicaid reimbursement policy with best evidence, and aimed to diminish the role of long-term use of opioid medication in treatment of chronic neck and back pain while emphasizing less harmful treatments.
Our cohort of patients had a high level of physical and mental health comorbidity. This is expected in CHCs that are known to serve more complex populations21 and especially among a subset of patients with chronic pain. Nor is it unexpected to see lower baseline opioid doses among LOT patients in CHCs compared with those reported in other population descriptions.6,14 A previous analysis of opioid prescribing in the OCHIN network in 17 states showed that prescribing per capita decreased from 1,682 MME in 2009 to 243 MME in 2018.28 This was compared with an estimated decrease from 733 MME to 424 in the same years for the entire nation.6 In addition, an OHP policy in 2012 required prior authorization for opioid doses over 120 MME/day,29 and a 2015 to 2017 statewide performance improvement program encouraged dosage reduction to below 90 MME daily.30
The Oregon Back and Neck Pain revised guidelines became effective on July 1, 2016. By the target discontinuation date, January 1, 2018, roughly half of the patients in our study cohort were no longer being prescribed opioids. Another 30% were being prescribed less than they had 18 months earlier, and only 20% had maintained or increased their dose. This preponderance of tapering and discontinuation occurred regardless of baseline dose. Even among those whose baseline doses exceeded 90 daily MMEs, nearly half fully discontinued and the majority of the rest tapered to safer doses. Notably, only 6% of this highest-risk group did not decrease their dose at all.
Several studies have explored opioid dose reduction in patient cohorts after interventions. For example, in a cohort of 50 Veterans Administration LOT patients who agreed to be part of a tapering program, 12% fully discontinued opioids, 82% partially tapered, and 6% were on the same or higher dose after 1 year.31 Another initiative in an academic medical center clinic was designed to enforce a maximum opioid dose of 120 MME/day. Of the 116 identified patients above this cutoff, 40% were able to taper to less than 120 MMEs/day by the 1-year target.32 A third study evaluated a program in a large health care system in which primary care clinicians identified patients they thought would benefit from an intensive tapering program. Of 1,384 patients who consented and were referred, 56% reduced their dose by 50% after 1 year.14 In light of these studies, our finding that 50% of our cohort fully discontinued opioid use and another 30% partially tapered is noteworthy.
The strength with which the pendulum swung back against using opioids for chronic pain in the 2010s raised concerns that policies aimed at decreasing opioid prescribing could lead to abrupt discontinuation, withdrawal and iatrogenic harm.16,17 The CDC Guideline published in 2016 suggested that, once having made the decision to taper, decreases of 10% weekly might be a good starting point, and slower tapers (10% monthly) more appropriate for some. Recent studies have shown that these concerns were indeed warranted, and that tapering often occurs more rapidly than 10% weekly.33⇓–35 However this does not seem true of the OHP policy implementation we describe. As seen in Table 2 and Figure 2, those who fully discontinued opioid use tapered over 9 or more months, with those on higher baseline doses taking longer to do so. These data provide compelling evidence that, at least within Oregon CHC settings, tapering and discontinuation did, indeed, occur gradually.
In our cohort, those who discontinued their opioids were more likely to be younger, less medically complex, have a diagnosis of opioid use disorder, and be non-Hispanic White (Table 3). We found no difference by sex, or by the presence of depression or anxiety. Most other studies that characterize patients by likelihood of opioid tapering have found similar association with younger age.14,33 Unlike our findings, others have reported that patients are more likely to taper if they are female,14,33,36 more medically complex,14,33 or Black.33,36 Unfortunately, given the relatively small population of Black patients in Oregon and thus, in our Oregon Medicaid cohort, we had insufficient sample size to specifically explore the association of a patient's race or ethnicity with opioid discontinuation. Given well-known racial disparities with regard to pain treatment,37,38 the experience of Black patients with regard to opioid tapering warrants further study.
Our study has a number of limitations. First, our EHR data contains orders for opioid prescriptions initiated from a network of CHCs. It is not possible to determine if these prescriptions were picked up or ingested by patients, nor would our data capture opioid prescriptions obtained from other clinical settings. Second, while all subjects had back or neck pain and were taking LOT, we do not know that the opioid prescriptions were meant specifically to treat their back or neck pain. Third, some patients who seem to have discontinued opioid use may have been misclassified because of not returning for follow-up visits to ascertain further prescribing. Similarly, if patients changed care to a non-OCHIN clinic during the observation period, any subsequent opioid prescribing would be unobserved. However, our sample selection of established patients receiving long-term opioid therapy for at least 6 months in this clinical setting at baseline, and requiring at least 1 visit in the study period, limited the potential effects of this bias. Only about one third of patients did not have any further visits after their last opioid prescription. Our sensitivity analysis excluding these “lost-to-follow-up” patients did not change overall conclusions. Finally, as noted above, there have been many interventions at all levels over the past decade to reduce inappropriate opioid prescribing. We cannot know with certainty how much of the effect we describe in our cohort was due to this specific Oregon Medicaid policy. Yet, given the size of the decline, its consistency among groups, and the stable nature of the clinical population, we believe that at least some component of the opioid discontinuation we have described is due to the policy. This study is part of a larger mixed-methods research project evaluating the policy using a natural experiment design; analysis of other data sets (including California Medicaid data as control group) will help further answer this question.22
Conclusion
In recent years, efforts to mitigate the harms of LOT in patients and communities required enacting policies with incomplete evidence about potential outcomes.7,18,39 Our study shows that, regardless of baseline dose, nearly half of the patients affected by Oregon Medicaid policies concerning treatment of back and neck pain no longer received opioid prescriptions at the end of the study period, while another 30% partially tapered their dose. Moreover, our data show there was very little abrupt discontinuation. Further research is needed to understand the broader impact of such insurance reimbursement policy changes, and especially individual patient experiences and outcomes to help clinicians and policy makers navigate the complex balance between potential harms and potential benefits of chronic opioid therapy.
Acknowledgments
This work was supported by the Accelerating Data Value Across a National Community Health Center Network (ADVANCE) Clinical Research Network (CRN). OCHIN leads the ADVANCE network in partnership with Health Choice Network, Fenway Health, and Oregon Health & Science University. ADVANCE is funded through the Patient-Centered Outcomes Research Institute (PCORI), contract number RI-CRN-2020-001.
Appendix
Condition Definitions
CHC Utilization during 18-Month Study Follow-Up Period by Ending MME Dose
Baseline Characteristics, Full Sample, and Sensitivity Sample Excluding Complete Discontinuation Patients with No Further Clinic Visit Following Last Opioid Prescription
Opioid Reduction Outcomes, Sensitivity Sample Excluding Complete Discontinuation Patients with No Further Clinic Visit Following Last Opioid Prescription
Notes
This article was externally peer reviewed.
Conflicting and competing interests: Dr. Livingston previously served as the Associate Medical Director of the Health Evidence Review Commission. She is currently employed as the Medical Director of Health Share of Oregon. This potential conflict of interest has been reviewed and managed by Oregon Health & Science University.
Dr. Shortreed has been a co-Investigator on Kaiser Permanente Washington Health Research Institute (KPWHRI) projects funded by Syneos Health, that is representing a consortium of pharmaceutical companies carrying out FDA-mandated studies regarding the safety of extended-release opioids.
Funding: This work was supported through a PCORI Project Program Award (UOP-1609-36568).
To see this article online, please go to: http://jabfm.org/content/35/2/352.full.
- Received for publication July 16, 2021.
- Revision received September 25, 2021.
- Accepted for publication September 29, 2021.