Abstract
Platelet activation and aggregation plays an integral role in the pathogenesis of acute coronary syndrome (ACS). The mainstay of ACS treatment revolves around platelet inhibition. It is known that greater platelet inhibition results in better ischemic outcomes; hence, focus in drug development has been to create more potent inhibitors of platelet aggregation. Prasugrel, a potent, third-generation thienopyridine, was approved by the US Food and Drug Administration in July 2009 for its use in ACS and percutaneous coronary intervention. The addition of prasugrel to aspirin for dual antiplatelet therapy has been shown to reduce the ischemic outcomes compared with clopidogrel and aspirin in combination. However, being a more potent antiplatelet agent, prasugrel increases the risk of bleeding, especially in those patients who are at a higher risk of bleeding complications. Elderly patients ≥75 years, patients who weigh ≥60 kg, and patients with a history of stroke or transient ischemic attack are at a higher risk of bleeding complications when prasugrel is used in combination with aspirin. Newer antiplatelets currently are being clinically evaluated to assess their efficacy in reducing ischemic events without increasing the bleeding risk.
Chest pain and acute coronary syndrome (ACS) are major diagnoses for hospital admissions and as single diagnoses they remain major determinants of health care cost. It is important that the high incidence of ACS is also associated with high mortality. The American Heart Association reports that ACS affects one American every 25 seconds and of those, one person dies every minute. It is estimated that 785,000 people in United States will have a new coronary event in 2010.1 Hence, targeting strategies to improve the outcome related to this condition has been the primary focus in the last 20 to 30 years. Most of the developments in pharmacotherapy have been in targeting platelet inhibition because platelet aggregation and the resultant formation of platelet-rich thrombi are the primary events in the pathogenesis of ACS.
Platelet Function and Antiplatelet Agents
Platelet adhesion, activation, and aggregation play an integral role in the pathogenesis of ACS. Compared with red cell thrombi, platelet-rich thrombi are difficult to lyse and notoriously promote development of reocclusion.2 Therefore, antiplatelet therapy is indispensible in the early and long-term management of ACS.
Aspirin, which exerts its antiplatelet effects by inhibiting thromboxane A2 production, has been the mainstay of antiplatelet therapy in patients with ACS. However, suboptimal clinical outcomes with aspirin monotherapy resulted in the use of dual antiplatelet therapy with the addition of thienopyridines. Thienopyridines are a group of drugs whose metabolites bind irreversibly to adenosine diphosphate (ADP) receptors on platelets (P2Y12 receptors), resulting in the inhibition of ADP-induced platelet activation and aggregation. The irreversible binding to the receptors makes these drugs capable of inhibiting platelet function for the life span of platelets (5–7 days). Thienopyridines include ticlopidine (first generation), clopidogrel (second generation), and the most recent addition, prasugrel (third generation). The latest class of antiplatelet agents, called cyclo-pentyl-triazolo-pyrimidines, includes ticagrelor and cangrelor. The US Food and Drug Administration (FDA) approved ticagrelor for clinical use in July 2011, but cangrelor is not yet approved. These drugs, unlike thienopyridines, bind reversibly, but directly without biotransformation, to P2Y12 receptors on platelets and hence inhibit platelet function. The antiplatelet action of both of these drugs is reversible within hours of discontinuation of therapy.3
Aspirin alone or in combination with a thienopyridine (dual antiplatelet therapy) reduces the risk of coronary ischemic events in patients with ACS.4,5 Nevertheless, catastrophic ischemic events still occur, especially in high-risk patients such as those who undergo percutaneous coronary intervention (PCI). This led to the introduction of the third-generation thienopyridine prasugrel for dual antiplatelet therapy for ACS management. This article focuses on prasugrel, which was approved by the FDA in July 2009 for use in moderate- to high-risk patients with ACS who undergo PCI.
Prasugrel as an Antiplatelet Agent
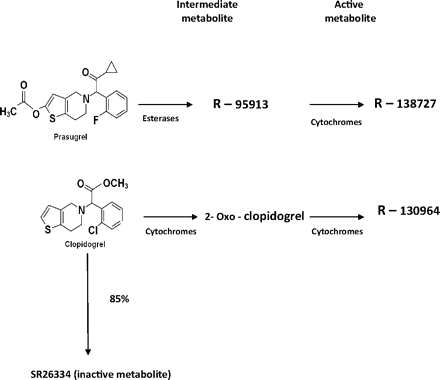
Thienopyridines are converted to active metabolites (Figure 1) that bind irreversibly to P2Y12 receptors on platelets and thereby inhibit ADP-induced platelet activation and aggregation. Ticlopidine, the first thienopyridine to be introduced, stepped out of the limelight largely because of its unacceptable side effects (aplastic anemia and thrombotic thrombocytopenic purpura).6 Clopidogrel, which has a better side-effect profile, soon replaced ticlopidine as the preferred antiplatelet agent in dual antiplatelet therapy with aspirin. Clopidogrel therapy has been shown to improve outcomes in patients with ACS and in those undergoing emergent or elective PCI.7,8 Clinical trials also have shown that combining clopidogrel with aspirin resulted in an additional 20% reduction in nonfatal myocardial infarction, stroke, and death compared with aspirin alone.9 However, clopidogrel has some limitations, such as interpatient variability in antiplatelet response and delayed onset of action.10 In this context, prasugrel, a third-generation thienopyridine with better antiplatelet activity and faster onset of action, has gained importance.
Metabolic pathways of clopidogrel and prasugrel.
Prasugrel is a prodrug, like its earlier congeners ticlopidine and clopidogrel. It is absorbed quickly and is converted into its active metabolite in a 2-step process involving hydrolysis by esterases and then oxidation by multiple cytochrome P450 isotypes. The active metabolite attains peak plasma concentration within 30 minutes of administration of prasugrel and is excreted predominantly by the kidneys.11
Clopidogrel, on the other hand, is extensively deactivated by esterases (∼85% of administered drug), thereby reducing its bioavailability. Also, the conversion of clopidogrel to its active metabolite is largely dependent on a specific cytochrome, P3A4.5 It is, therefore, not surprising that the active metabolite of clopidogrel attains peak plasma concentration in only about an hour after administration of clopidogrel. In both prasugrel and clopidogrel, the respective active metabolites bind to the P2Y12 receptors on platelets and exert antiplatelet activity. Hence, it is logical that the drug that generates the active metabolite faster will have a more rapid onset of action.
Niitsu et al12 showed that dual antiplatelet therapy with aspirin and prasugrel produced better antiplatelet activity than either agent used alone. Prasugrel is approximately 10- and 100-fold more potent in inhibiting platelet function in vivo than clopidogrel and ticlopidine, respectively.13 A 60-mg loading dose of prasugrel followed by a 10-mg daily maintenance dose resulted in greater level of inhibition of platelet aggregation at 4 hours compared with a 300-mg loading dose of clopidogrel with a 75-mg daily maintenance dose (68.4% vs 30%, respectively). In addition, the pharmacologic nonresponders were fewer with prasugrel compared with clopidogrel (3% vs 52%, respectively).14 Clinical trials have proven that prasugrel produces faster antiplatelet activity and lesser interpatient variability when compared with clopidogrel.15,16 In light of this, compared with clopidogrel, prasugrel is suggested to have a faster onset of action, greater potency, and lesser individual variability in inhibition of platelet function.17 A faster and more efficient generation of the active metabolite is suggested to be the reason for these attributes of prasugrel.
Clinical Trial With Prasugrel
The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 3818 compared the use of prasugrel (60-mg loading dose and maintenance dose of 10 mg/day) with clopidogrel (300-mg loading dose followed by a daily 75-mg maintenance dose) in 13,608 patients with moderate to high risk of ACS for whom PCI was scheduled. All patients received aspirin at a dose of 81 mg daily. Patients were followed-up over a period of 6 to 15 months. Results showed that patients treated with prasugrel had lower rates of myocardial infarction (9.7% for clopidogrel vs 7.4% for prasugrel; P < .001), urgent target-vessel revascularization (3.7% vs. 2.5%; P < .001), and stent thrombosis (2.4% vs. 1.1%; P < .001). Congruent with the higher antiplatelet activity, the prasugrel group also had higher major bleeding rates (2.4% vs 1.8%; hazard ratio [HR], 1.32; 95% CI, 1.03–1.68; P = .03). But, when a prespecified analysis assessing the net clinical benefit was done including all cause mortality, stroke, myocardial infarction, and major bleeding, the results favored prasugrel (13.9% of patients in the clopidogrel group vs 12.2% in the prasugrel group). Three high-risk subgroups with lesser net clinical efficacy and higher rates of bleeding were found in a post hoc analysis: patients ≥75years old, patients weighing ≤60 kg, and patients with a history of stroke or transient ischemic attack.
Patients with diabetes mellitus (DM) had greater benefit compared with nondiabetic patients when dual antiplatelet therapy with prasugrel was used. In a subanalysis of the TRITON-TIMI 38 study, prasugrel use was more efficacious compared with clopidogrel in reducing the ischemic event rates in both diabetics and nondiabetics, with greater reduction in diabetics.19 The rate of primary endpoint among patients without DM was 9.2% versus 10.6% (HR, 0.86; P = .02) and was 12.2% versus 17.0% among patients with DM (HR, 0.70; P < .001). The TIMI major hemorrhage rates were similar among patients with DM for clopidogrel and prasugrel (2.6% vs 2.5%; HR, 1.06; P = .81). This suggests that the greater antiplatelet activity produced by prasugrel resulted in higher net clinical benefit in patients with DM when compared with patients without DM.
Similarly, in another subanalysis of TRITON-TIMI 38, prasugrel was found to be more efficacious in reducing ischemic events in both drug-eluting stent and bare metal stent patient groups compared with clopidogrel.20 Prasugrel also reduced the incidence of in-stent thrombosis rates in patients with either drug-eluting stent implantation (0.84 vs 2.31%; HR 0.36; P < .0001) or in those with bare metal stents (1.27 vs 2.41%; HR 0.52; P = .0009).20 Also, prasugrel was shown to have an absolute risk benefit in preventing stent thrombosis in patients with diabetes, bifurcation stents, and longer stents (>2 cm).
The use of prasugrel in ACS patients undergoing PCI has been shown to be beneficial and cost-effective compared with the use of clopidogrel21 when combined with aspirin (strength of recommendation, B; level of evidence, 1). The efficacy of prasugrel monotherapy in this patient group has not been studied. The approved dosage of prasugrel is a 60-mg loading dose followed by a 10-mg daily maintenance dosage. Dose adjustment is not required in patients with renal dysfunction; however, safety of prasugrel in patients with end-stage renal failure has not been well established.
Effects of Concomitant Proton Pump Inhibitor Use and Other Drug Interactions
During antiplatelet therapy, many patients develop gastrointestinal symptoms that often require the addition of one of the proton pump inhibitors (PPIs). Studies have suggested a potential adverse drug interaction between clopidogrel and PPIs, causing a reduction in the efficacy of clopidogrel and thereby an increased incidence of ischemic events.22 In light of this evidence, in November 2009 the FDA announced a public health warning regarding the possible interaction between clopidogrel and PPIs. In a single-center, open-label, randomized study, Small et al23 concluded that concurrent use of lansoprazole and prasugrel did not decrease the inhibition of platelet aggregation by prasugrel, whereas lansoprazole lowered the level of inhibition of platelet aggregation when used concurrently with clopidogrel. Interestingly, an analysis of 2 randomized trials by O'Donoghue et al24 showed that, when clinically indicated, PPIs can be used in patients taking either clopidogrel or prasugrel. Hence, conflicting evidence of the safety of the concurrent use of clopidogrel and PPIs still exists, but all available evidence about the concurrent use of prasugrel and PPIs points toward relative safety.
There is no drug interaction with inducers or inhibitors of cytochrome P450 enzymes. Therefore, prasugrel can be used in patients already receiving treatment with rifampicin, carbamazepine, ketoconazole, verapamil, diltiazem, indinavir, ciprofloxacin, and clarithromycin and in patients taking grape fruit juice. Concomitant use with heparin, warfarin, fibrinolytic agents, and glycoprotein IIb/IIIa receptor antagonists will increase the risk of bleeding.
Summary
Antiplatelet agents play an important role in the management of ACS. Along with aspirin, many new antiplatelet agents including thienopyridines have been used clinically. All thienopyridines undergo biotransformation to active metabolites, which bind to P2Y12 receptors on platelets, resulting in inhibition of ADP-mediated platelet activation and aggregation.5 Prasugrel, a novel thienopyridine, has been shown to have faster and more complete antiplatelet action in vivo compared with other thienopyridines.13 It also has lesser inter patient variability in its antiplatelet effects when compared with clopidogrel. These desirable effects are because of better absorption and a more efficient metabolism. See Table 1 for a comparison of different antiplatelet agents.
TRITON-TIMI 38 showed that prasugrel therapy lowered the rate of ischemic events in moderate- to high-risk patients with ACS who were scheduled for PCI. This better antiplatelet effect comes, however, at the cost of an increase in major bleeding, especially among 3 high-risk groups: patients ≥75 years old, patients weighing ≤60 kg, and patients with a history of stroke or transient ischemic attack. It would be prudent to avoid the use of prasugrel in these groups of patients if possible.18 It has been suggested that in the former 2 groups, lowering the prasugrel dosage from the standard 10 mg/day to 5 mg/day might reduce the incidence of bleeding.25 Also, increased bleeding was observed in patients taking prasugrel who were undergoing coronary artery bypass graft when compared with patients taking clopidogrel.
Prasugrel is a good choice for patients with DM because they have increased platelet reactivity26 and higher rates of nonresponse to clopidogrel.27 Prasugrel has been shown to improve net outcomes among patients with DM rather than in those without DM.19 Although there is conflicting evidence about concurrent use of clopidogrel and PPIs, all available evidence suggests that PPIs can be safely used in patients taking prasugrel. In TRITON-TIMI 38, the incidence of stent thrombosis was lower in patients treated with prasugrel when compared with those treated with clopidogrel. It is noteworthy that results from TRITON-TIMI 38 cannot yet be applied to all ACS patients because this study looked into only patients with ACS who were scheduled for PCI. Although prasugrel seems promising, as of now its use in all patients with ACS is not advisable.
Bleeding, including life-threatening and fatal bleeding, is the most commonly reported adverse reaction of prasugrel use. Occurrence of stroke or transient ischemic attack while taking prasugrel would warrant discontinuation of the drug. Active bleeding and elective surgery are the other reasons for discontinuation of the drug. A rare but serious and potentially fatal condition, thrombotic thrombocytopenic purpura, has been reported with prasugrel. This sometimes can occur after a brief exposure (<2 weeks) and requires urgent treatment, including plasmapheresis.
Future Directions
Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes28 is a phase 3 trial, expected to be completed in April 2012, that will compare prasugrel therapy with clopidogrel therapy among patients with ACS who are medically managed and in whom no revascularization is planned. This study is expected to throw light on the efficacy and safety of prasugrel when used in a different spectrum of ACS patients.
The next antiplatelet agents in the pipeline are ticagrelor and cangrelor. Both of these are direct inhibitors of P2Y12 receptors on platelets and cause reversible inhibition of platelet function. Neither of these drugs require biotransformation before they can act and are thus different from thienopyridines in use now. Cangrelor is administered intravenously and ticagrelor is an orally active drug. Cangrelor is metabolized in the plasma and starts exerting its antiplatelet action within seconds of beginning intravenous infusion. Once the infusion is stopped, the platelet activity reverts back to normal within an hour. These characteristics would make it a major competitor to glycoprotein IIb/IIIa inhibitors and a favorite drug for interventional cardiologists once its use is approved by the FDA. CHAMPION PCI is a phase 3 trial that will investigate the proposed superiority of cangrelor over clopidogrel in reducing ischemic events when given at the start of PCI. The PLATO trial proved the superior efficacy of ticagrelor over clopidogrel in patients with ACS.29
Looking back, we find that, after aspirin, new drugs with better antiplatelet activity have been added at regular intervals. This trend probably will continue and hopefully lead to the development of better antiplatelet agents.
Notes
This article was externally peer reviewed.
Funding: none.
Conflict of interest: none declared.
- Received for publication November 12, 2010.
- Revision received October 17, 2011.
- Accepted for publication October 25, 2011.